![]()
chapter one
Molecular substrates of ethanol feedstocks
Regardless of the type of feedstock, all bioethanol production processes rely on isolating one or more molecular substrates that are used to generate precursors for ethanol-producing reactions. These molecular substrates include starch, sucrose, cellulose, hemicellulose, lignin, and pectin—which may be further decomposed for use (or used directly) by yeast in ethanol fermentation. Whereas the role of yeast in ethanol fermentation is a well-studied component of the overall process, the conversion of these molecular substrates to readily fermentable sugars (i.e., glucose) is a subject of ongoing research and is often considered a bottleneck in bioethanol production. Indeed, the culturing of yeast and ethanol production are typically two tightly coupled processes used in fermentation via glycolysis, the Embden–Meyerhof–Parnas pathway (Madigan et al., 2000). Glycolysis ultimately yields two pyruvate molecules for each molecule of glucose that is processed. Under anaerobic conditions, pyruvate is subsequently reduced to ethanol with a release of CO2. Thus, enhancing processes that decompose complex molecular substrates to glucose or other fermentable sugars is a key to increasing overall bioethanol production efficiency.
1.1 Starch and sucrose
Historically, the production of ethanol has relied almost exclusively on two molecular substrates: starch and sucrose from corn (Zea mays L.) and sugarcane (Saccharum L.), respectively. More broadly, starches from corn and wheat and reducing sugars from sugarcane and sorghum are extracted, typically via mechanical processes and serve as substrates for subsequent ethanol fermentation steps (Nichols and Bothast, 2008; Dias et al., 2009).
1.1.1 Starch
Corn is milled to extract starch. There are two types of corn milling in the industry: wet and dry. During wet milling, the corn grain is pulverized and then separated into its various components: starch, fiber, gluten, and germ. On separating the starch as a solution from other components, it is typically treated with enzymes to generate simpler sugars that are easily fermented by yeast. The remaining components of the wet-milling process are often sold separately as coproducts. In wet milling, grains are steeped in dilute sulfuric acid prior to separation of grain components. However, in dry milling, whole grain meal is directly fermented to produce ethanol and by-products collectively called distiller’s dried grains with solubles (DDGS), the latter of which may be used for animal feed or extracted for oil, which in turn may be converted to biodiesel via transesterfication (Singh and Cheryan, 1998; Bothast and Schlicher, 2005).
Although initial treatment of corn grain and the types of by-products that are generated differ between the two processes, both wet and dry milling ultimately break down starch via enzymatic processes, convert sugar to ethanol releasing CO2 using yeast, and refine/dehydrate the resulting ethanol for use as fuel. In corn-based bioethanol production, starch is an abundant and readily hydrolysable substrate. Indeed, starch is the major chemical component within the corn kernel. It accounts for ~72% of kernel weight with another ~2% consisting of sugars such as glucose, sucrose, and fructose (Inglett, 1970). Cornstarch is generally composed of two types of polymers: amylose and amylopectin. Amylose is essentially a linear polymer of glucose. Amylopectin is a branched glucose polymer.
Of the total starch content in corn kernel, about 20%–25% is amylose and 75%–80% is amylopectin (Schoch, 1942; Bates et al., 1943). However, yeast is not generally capable of metabolizing starch (Stewart and Russell, 1987; De Mot, 1990). Thus, amylose and amylopectin must be hydrolyzed. Several enzymes, including thermostable α-amylases, are used to break down starch into smaller molecules (i.e., dextrins), which are then enzymatically converted to glucose (a.k.a., dextrose) by various glucosidases (e.g., amyloglucosidase) in a process called saccharification (Bothast and Schlicher, 2005). As over 90% of ethanol production in the United States uses starch as a primary molecular substrate, the importance of thermostable and high-efficiency starch reducing enzymes is understood.
1.1.2 Sucrose
In contrast to cornstarch, sucrose is the principal molecular substrate in sugarcane-based bioethanol production. Sucrose is found in high concentrations in both cane juice and molasses (a by-product of sugar mills). It is the only major ethanol substrate that does not require industrial enzymatic processing. In other words, yeast can directly metabolize sucrose to produce ethanol. In this respect, sucrose has a major advantage as a molecular substrate in ethanol production.
Ethanol production using substrates from sugarcane begins with cleaning the feedstock followed by mechanical extraction and separation of juice, molasses, and bagasse. Sugarcane juice contains water, sucrose, and other reducing sugars. It also contains impurities such as minerals, salts, organic acids, dirt, and fiber particles, which must be removed before fermentation (Dias et al., 2009). Clarification and crystallization steps can be used to extract sugar crystals leaving behind molasses (Ensinas et al., 2009). For a 3 lb sugarcane stalk, approximately 0.3 lb of sugar is generated, and 100 lb of raw sugar yields about 3 gal of molasses. From each gallon of molasses, 0.41 gal of ethanol can be produced. Although both products can be used to produce ethanol, only the molasses is often used. However, if both the raw sugar and molasses are converted to ethanol, approximately 19.6 gal of ethanol can be produced per ton of harvested sugarcane (Shapouri et al., 2006).
Most industrial strains of yeast express invertases (e.g., sucrases) that convert sucrose to simple sugars (i.e., glucose and fructose), which are in turn readily converted to ethanol (and CO2) (Berthelot, 1860; Dworschack and Wickerham, 1961; Bowski et al., 1971). Thus, none of the early processing (e.g., liquefaction) that is required for using cornstarch as a molecular substrate is required for ethanol production that uses sucrose as the principal substrate. This saves several steps in the ethanol production process. The latter stages of fermentation and ethanol recovery are similar in both sugarcane-based and corn-based bioethanol production. Distillation and dehydration are used to purify the ethanol following fermentation by yeast (Bothast and Schlicher, 2005; Ensinas et al., 2009), and the remaining stillage from the distillation process can be recycled as fertilizer for sugarcane fields. Although sucrose is a competitive molecular substrate for bioethanol production, the feedstock from which it is derived (i.e., sugarcane) has production limitations as described in Section 2.1.
1.2 Cellulosic substrates
One of the most promising molecular substrates for bioethanol production is lignocellulose. Cellulose is the most abundant biopolymer on the planet. It accounts for approximately half of all biomass contained within photosynthetic plant matter. However, lignocellulosic materials are often bound in very recalcitrant matrices of cellulose, hemicellulose, lignin, and other molecules such as pectin (Table 1.1), which together render these materials unviable as competitive substrates due to the costs associated with accessing and breaking down these components into fermentable sugars. Nonetheless, emerging technologies that overcome this technical problem may soon elevate lignocellulose to a major substrate for industrial scale ethanol production.
Table 1.1 Chemical composition of cellulosic biomass (% composition)
Constituents | Hardwood (%) | Softwood (%) |
Cellulose | 40–50 | 40–50 |
Hemicellulose | 25–35 | 25–30 |
Lignin | 20–25 | 25–35 |
Pectin | 1–2 | 1–2 |
Starch | Trace | Trace |
Source: Miller, R.B., Structure of wood, in Wood Handbook: Wood as an Engineering Material, USDA Forest Service, Forest Products Laboratory, Madison, WI, 113, 1999.
1.2.1 Cellulose
Cellulose comprises at least 30%–50% of the total biomass in most lignocellulosic materials (Wyman, 1994; Mielenz, 2001; Mood et al., 2013). Cellulose is a linear, unbranched homopolysaccharide composed of β-D-glucopyranose units linked by β-1,4 glycosidic bonds (Jorgenson, 1950; Mark and Tobolsky, 1950; Hon, 1994). Chemically, cellulose is simply a chain of glucose molecules. However, structurally, the repeating unit is the disaccharide cellobiose (Staudinger, 1961). Individual cellulose chains can contain 102 to 104 glucose units. These chains are tightly packed into microfibrils with extensive hydrogen bonding as well as van der Waals interactions within and between cellulose chains, which provides stability to this high-order fibrous structure (Marchessault and Sarko, 1967; Cousins and Brown, 1995; Nishiyam et al., 2010).
Depending on the source and conditions of formation, cellulose will often form a crystalline structure; however, amorphous cellulose may also form a crystalline structure. With respect to the former, there are six distinct crystalline forms. Cellulose I (natural cellulose) and cellulose II (regenerated and mercerized cellulose) have been the most extensively studied (Habibi et al., 2010). Although crystalline cellulose can be difficult to degrade, amorphous cellulose tends to be more susceptible to enzymatic degradation (Hall et al., 2010). In either form, natural materials containing cellulose typically contain other polymers including hemicellulose, lignin, and pectin, which complicate the deconstruction of cellulose into glucose or other readily fermentable sugars.
1.2.2 Hemicellulose
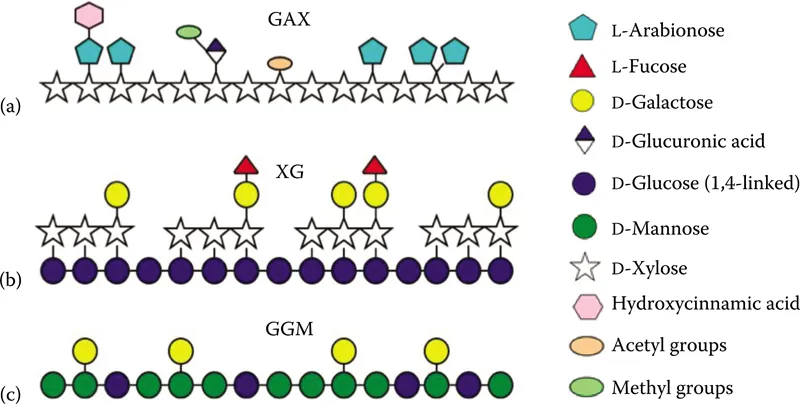
Hemicellulose comprises approximately 15%–35% of the total biomass in lignocellulosic materials (Wyman, 1994; Gírio et al., 2010; Mood et al., 2013). Hemicellulose is a branched heteropolymer of hexose sugars, pentose sugars, and sugar acids. Of these constituent components, D-mannose, D-glucose, and D-galactose are common hexoses; D-xylose and L-arabinose are common pentoses; and D-glucuronic acid, D-galacturonic acid, 4-O-methylglucaronic acid, and rhamnose are common sugar acids found within hemicellulose. Sugars are linked by β-1,4 bonds and β-1,3 glycosidic bonds. The former dominates most hemicellulosic structures with the main backbone typically composed of only one or two types of sugar. For example, xylose, in the form of 1,4-β-D-xylopyranose structural units, forms the homopolymeric backbone of structures called xylans. As significant branching from this backbone occurs (typically consisting of short chains of other sugars, acetyl groups, or phenolic groups), most xylans are ultimately heteropolymers. Xylans can be classified as linear xylans, heteroxylans, or xyloglucans (XGs). Linear xylans consist of 1,4-β-D-xylopyranose units linked to form a polymeric backbone. However, xylans often contain additional components including arabinose, glucuronic acid, feruloyl acid, or coumaroyl acid side chains and are thus heteroxylans. Based on backbone and branching motifs, heteroxylans can be subclassified as arabinoxylans, glucuronoxylans, and other combinatory species, such as glucuronoarabinoxylans (GAXs) (Schulze, 1891; Scheller and Ulvskov, 2010). The frequency and composition of branches depend upon the source of xylan (Gorbacheva and Rodionova, 1977; Aspinall, 1980; Fincher and Stone, 1981; Brice and Morrison, 1982; Scheller and Ulvskov, 2010). In Figure 1.1, the heterogeneity of selected xylan structures is shown.
Figure 1.1 Plant cell wall polysaccharides and structural heterogeneity. (a) Backbone structure of heteroxylan, (b) Backbone structure of XG, and (c) Representative structure of a heteromannan molecule. (Adopted and modified from Burton, R.A. et al., Nat. Chem. Biol., 6, 724–732, 2010.)
Acetyl groups and glucuronic acid moieties are found in hardwood hemicellulose (Timell, 1960; Bouveng, 1961; Timell, 1967; Gabrielii et al., 2000; Teleman et al., 2002). Arabinofuranosides and glucuronic acids are found in hemicellulose of softwoods and grasses (Marchessault et al., 1963; Timell, 1967; Shimizu et al., 1978; Wende and Fry, 1997; Verbruggen et al., 1998b; Pastell et al., 2009; Peng et al., 2011; Escalante et al., 2012). These heteroxylan (GAX) motifs are illustrated in Figure 1.1a. XG is a cellulosic (1,4)-β-glucan backbone supporting α-D-xylose side chains with about 70% occupancy of the backbone glucosyl residues linked at the glucose C-6 position. Triplet xylosyl substituents on three consecutive glucosyl residues are common along with additional side chain components such as galactose, fucose, or arabinose (Stephen, 1989; Sims et al., 1996; Hoffman et al., 2005; Tiné et al., 2006). A representative XG is shown in Figure 1.1a (side chain arabinose not shown).
Although xylans are the major components of hemicellulose, the heterogeneity of hemicelluloses should not be understated. Hemicelluloses are mixtures of polysaccharides and often include other components. Although hardwoods, grasses, and many fruits feature a robust presence of xylans, mannans play a major role in the hemicellulose found in softwoods and plant seed (Wilkie, 1979; Meier and Reid, 1982; Vierhuis et al., 2000; Lundqvist et al., 2003; Gírio et al., 2010). Mannans can be classified as linear mannan (e.g., glucomannan) or heteromannan (i.e., galactoglucomannan [GGM]). Glucomannan are linear chains composed of randomly arranged β-(1,4)-linked D-mannose with β-(1,4)-linked D-glucose. The ratios of mannose and glucose depend on the origin of glucomannan (Timell, 1967; Northcote, 1972; Popa and Spiridon, 1998; Hongshu et al., 2002). Another form of linear mannan called galactomannan (GM) features 1,6-linked α-...