
eBook - ePub
Fundamentals of Engineering Thermodynamics
V. Babu
This is a test
Share book
- 424 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Fundamentals of Engineering Thermodynamics
V. Babu
Book details
Book preview
Table of contents
Citations
About This Book
This book deals with all the concepts in first level Thermodynamics course. Numerous examples are given with the objective of illustrating how the concepts are used for the thermodynamic analysis of devices.
Please note: T&F does not sell or distribute the Hardback in India, Pakistan, Nepal, Bhutan, Bangladesh and Sri Lanka
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Fundamentals of Engineering Thermodynamics an online PDF/ePUB?
Yes, you can access Fundamentals of Engineering Thermodynamics by V. Babu in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Civil Engineering. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
INTRODUCTION
Thermodynamics originated as a subject in mechanical engineering at the start of the industrial revolution when, steam generated from burning coal was utilized to run machinery. The quest to improve the efficiency of devices that convert heat into useful work led to the evolution of engineering thermodynamics. Today, thermodynamics is almost synonymous with mechanical engineering! The laws of thermodynamics, based on experiments, have now come to be regarded as being among the fundamental laws of the Universe. They are now applied in diverse areas such as financial markets, cosmology, computing, bio-technology and agriculture, to name a few. The second law has a particularly special place in our minds because it seems to explain so many occurrences in our daily life. For instance, everyone can relate to statements like For something to become cleaner something else must become dirtier and Left to themselves things tend to go from bad to worse from the popular Murphy’s laws. These may quite rightly be viewed as statements of the Principle of Increase of Entropy, which we will discuss later. For readers who are interested in the philosophical aspects of the laws of thermodynamics, I strongly recommend the two books by Peter Atkins that are given in the Suggested Reading list. He summarizes the three laws of thermodynamics in the following manner:
First law: Heat can be converted into work
Second law: But completely only at absolute zero
Third law: And absolute zero is unattainable!
1.1 Macroscopic approach
In engineering thermodynamics, we are concerned with the conversion of heat into useful work. In this context, we will try to answer the following questions:
▪ How much of the input heat is converted into work by the engine under consideration?
▪ Everything else remaining the same, what is the maximum possible work output?
▪ What are the factors that affect the performance of the engine and by how much?
The framework that we develop will not only be useful for analyzing engines that convert heat into useful work but also engines that utilize work to produce a useful effect (like, for instance, a refrigerator or a heat pump). In addition, it will also allow us to evaluate the performance of individual devices that make up the engine.
The analysis that we are interested in, utilizes a macroscopic or black box approach that ignores internal details. For instance, when we say that a certain amount of heat is transferred to or from a device, details regarding how exactly the heat transfer takes place is immaterial to a thermodynamic analysis. Similarly, when work (or power) is supplied to a compressor, details of how this is utilized in the compression process is immaterial. Details of how a turbine converts the enthalpy of a fluid into power are similarly immaterial to the analysis. Such details are the subject matter of courses on Heat Transfer and Fluid/Turbo Machines.
Furthermore, molecular level details are also ignored in the macroscopic approach. The working substance is assumed to be a single entity with a unique value for the properties - pressure, density, temperature and so on. Mixing and stirring processes are assumed to be macroscopic in nature and molecular effects in such processes are neglected.
1.2 Continuum hypothesis
An important requirement of the macroscopic approach that we have adopted here is that continuum must prevail. Only then, properties such as pressure, density, temperature and so on of the thermodynamic systems under consideration will be known without any ambiguity. In other words, a statement that the pressure of air in a vessel is a certain value, implicitly assumes that continuum prevails.
Consider the following thought experiment. A cubical vessel of a side dimension L contains a certain amount of a gas. One of the walls of the vessel has a view port to allow observations of the contents within a fixed observation volume. We now propose to measure the density of the gas at an instant as follows - count the number of molecules within the observation volume; multiply this by the mass of each molecule and then divide by the observation volume.
To begin with, let there be 100 molecules inside the vessel. We would notice that the density values measured in the aforementioned manner fluctuate wildly going down even to zero at some instants. If we increase the number of molecules progressively to 103, 104, 105 and so on, we would notice that the fluctuations begin to diminish and eventually die out altogether. Increasing the number of molecules beyond this limit would not change the measured value for the density.
We can carry out another experiment in which we attempt to measure the pressure using a pressure sensor mounted on one of the walls. Since the pressure exerted by the gas is the result of the collisions of the molecules on the walls, we would notice the same trend as we did with the density measurement. That is, the pressure measurements too exhibit fluctuations when there are few molecules and the fluctuations die out with increasing number of molecules. The measured value, once again, does not change when the number of molecules is increased beyond a certain limit.
We can intuitively understand that, in both these experiments, when the number of molecules is less, the molecules travel freely for a considerable distance before encountering another molecule or a wall. As the number of molecules is increased, the distance that a molecule on an average can travel between collisions (which is termed as the mean free path, denoted usually by λ) decreases as the collision frequency increases. Once the mean free path decreases below a limiting value, measured property values do not change any more. The gas is then said to behave as a continuum. The determination of whether the actual value for the mean free path is small or not has to be made relative to the physical dimensions of the vessel. For instance, if the vessel is itself only about 1 µm in dimension in each side, then a mean free path of 1 µm is not at all small! Accordingly, a parameter known as the Knudsen number (Kn) which is defined as the ratio of the mean free path (λ) to the characteristic dimension (L) is customarily used. Continuum is said to prevail when Kn ≪ 1. In reality, once the Knudsen number exceeds 10−2 or so, the molecules of the gas cease to behave as a continuum.
CHAPTER 2
BASIC CONCEPTS
In this chapter, certain basic concepts which are essential for carrying out a thermodynamic analysis of devices, are discussed. Although these concepts are relatively simple and easily understood by and large, they are discussed at length here in order to clearly bring out certain subtle aspects that are generally overlooked. A clear understanding of these basic concepts will form a strong foundation for the material developed later.
2.1 System
Following Spalding and Cole, we define a thermodynamic system as a quantity of matter of fixed mass and identity on which attention is focussed for study. Everything external to the system is referred to as the surroundings. Defining a system is a crucial first step in any thermodynamic analysis. In some cases, it may be relatively straightforward to define a system, while in some other cases, it may not be. Furthermore, a valid thermodynamic system need not necessarily be useful for analysis. It is possible to define more than one valid system for a given problem. The choice of which one to use is guided by the information given, the information sought and the ease of analysis. Let us explore these aspects through several examples.
We start with a very simple problem. A gas is contained within a piston cylinder assembly as shown in Fig. 2.1. Heat is added to the gas until it expands to a certain volume. We wish to define a system suitable for a thermodynamic analysis of this problem.
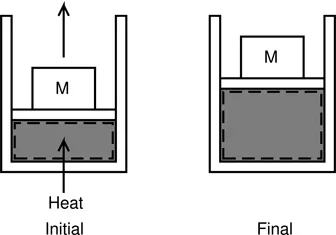
It is quite easy (perhaps trivial) to define a system for this problem. This is indicated as the shaded region in Fig. 2.1 and the system boundary is shown using a dashed line. The simplicity of the problem is deceptive and it is necessary to make the following observations:
▪ This system contains the same amount of matter from the beginning to the end of the process and thus satisfies the definition given above.
▪ During the process, parts of the system boundary adjacent to the walls of the cylinder remain fixed while that part adjacent to the piston moves along with it. In other words, the system boundary deforms in such a manner as to always contain the same mass throughout. Hence, it is essential to know the system boundary throughout the process - not just at the beginning and the end of the process. This is an important requirement since it implicitly demands that the process take place slowly. This also ensures that the pressure, temperature and volume are measurable at every instant and will be uniform throughout the system.
▪ Intuitively, it is easy to appreciate that wherever there is deformation of the system boundary, there is a work interaction between the system and the surroundings - either the system is doing work, as in this example, where the piston and the mass are being lifted and the atmosphere is being pushed upwards, or the surroundings do work on the system. The system boundary expands in the case of the former and contracts in the case of the latter. Such a work interaction is termed displacement work and an expression for evaluating the same is developed in the next chapter.
▪ It is possible to define other, equally valid systems for this problem. For instance, a system that contains the gas and the piston, or one that contains the gas, piston and the mass or one that contains just the atmosphere are all valid.
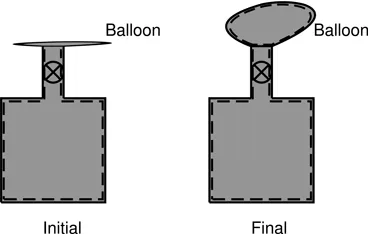
Our next example looks at inflating an initially empty balloon from a rigid vessel that contains air at a pressure higher than the atmospheric pressure. A suitable thermodynamic system for analyzing this problem is shown in Fig. 2.2. It is clear from this figure that this system contains the same amount of matter throughout and hence is a valid thermodynamic system. The part of the system boundary which is outside the vessel expands during the process, from which it may be inferred that the air in the vessel is doing work to expand the balloon. If the balloon material is thin and inextensible, then the pressure inside the balloon is the same as atmospheric pressure and the work is done entirely on the atmosphere. On the other hand, if the balloon material is elastic in nature (such as a rubber sheet), then the pressure inside the balloon will be higher than the atmospheric pressure and the work done by the air is partly utilized to stretch the balloon material and partly to push the atmosphere aside. In both cases, the process is guaranteed to take place slowly by the presence of the valve, which provides the required resistance. Unlike the previous example, here, different parts of the system, namely, the air inside the cylinder and the air inside the balloon, are at different pressures. At first sight, this may appear to violate the framework of the macroscopic approach that we have adopted here. This is not so, since the valve is a mechanical device that can support the pressure difference across these two parts of the system. Note that, in this example, the atmosphere...