
- 485 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Nanocrystal Quantum Dots
About this book
A review of recent advancements in colloidal nanocrystals and quantum-confined nanostructures, Nanocrystal Quantum Dots is the second edition of Semiconductor and Metal Nanocrystals: Synthesis and Electronic and Optical Properties, originally published in 2003. This new title reflects the book's altered focus on semiconductor nanocrystals.
Gathering contributions from leading researchers, this book contains new chapters on carrier multiplication (generation of multiexcitons by single photons), doping of semiconductor nanocrystals, and applications of nanocrystals in biology. Other updates include:
- New insights regarding the underlying mechanisms supporting colloidal nanocrystal growth
- A revised general overview of multiexciton phenomena, including spectral and dynamical signatures of multiexcitons in transient absorption and photoluminescence
- Analysis of nanocrystal-specific features of multiexciton recombination
- A review of the status of new field of carrier multiplication
- Expanded coverage of theory, covering the regime of high-charge densities
- New results on quantum dots of lead chalcogenides, with a focus studies of carrier multiplication and the latest results regarding Schottky junction solar cells
Presents useful examples to illustrate applications of nanocrystals in biological labeling, imaging, and diagnostics
The book also includes a review of recent progress made in biological applications of colloidal nanocrystals, as well as a comparative analysis of the advantages and limitations of techniques for preparing biocompatible quantum dots. The authors summarize the latest developments in the synthesis and understanding of magnetically doped semiconductor nanocrystals, and they present a detailed discussion of issues related to the synthesis, magneto-optics, and photoluminescence of doped colloidal nanocrystals as well. A valuable addition to the pantheon of literature in the field of nanoscience, this book presents pioneering research from experts whose work has led to the numerous advances of the past several years.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 “Soft” Chemical Synthesis and Manipulation of Semiconductor Nanocrystals
CONTENTS
1.1 Introduction
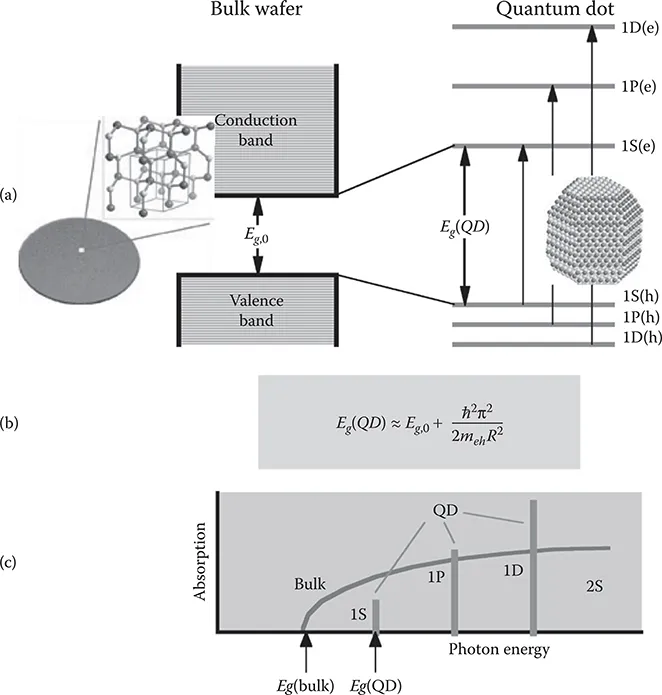
1.2 Colloidal Nanosynthesis
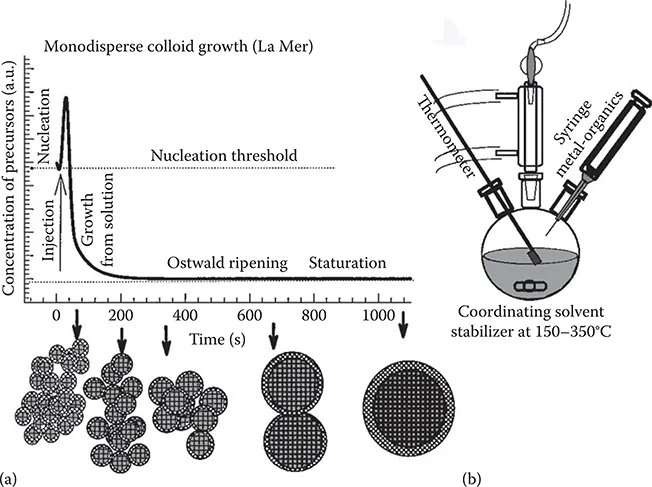
Table of contents
- Cover Page
- Title Page
- Copyright Page
- Table of Contents
- Preface to the Second Edition
- Preface to the First Edition
- Editor
- Contributors
- Chapter 1 “Soft” Chemical Synthesis and Manipulation of Semiconductor Nanocrystals
- Chapter 2 Electronic Structure in Semiconductor Nanocrystals: Optical Experiment
- Chapter 3 Fine Structure and Polarization Properties of Band-Edge Excitons in Semiconductor Nanocrystals
- Chapter 4 Intraband Spectroscopy and Dynamics of Colloidal Semiconductor Quantum Dots
- Chapter 5 Multiexciton Phenomena in Semiconductor Nanocrystals
- Chapter 6 Optical Dynamics in Single Semiconductor Quantum Dots
- Chapter 7 Electrical Properties of Semiconductor Nanocrystals
- Chapter 8 Optical and Tunneling Spectroscopy of Semiconductor Nanocrystal Quantum Dots
- Chapter 9 Quantum Dots and Quantum Dot Arrays: Synthesis, Optical Properties, Photogenerated Carrier Dynamics, Multiple Exciton Generation, and Applications to Solar Photon Conversion
- Chapter 10 Potential and Limitations of Luminescent Quantum Dots in Biology
- Chapter 11 Colloidal Transition-Metal-Doped Quantum Dots
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app