![]()
1
Introduction
1.1 Background
Plasma is often referred to as the fourth state of matter in which a certain portion of particles in gas or liquid is ionized. The term plasma was first introduced by Irving Langmuir; the way an electrified fluid carried electrons and ions moving at high velocity reminded him of the way blood plasma carried red and white corpuscles. In his article published in the Proceedings of the National Academy of Sciences in 1928, he wrote: “Except near the electrodes, where there are sheaths containing very few electrons, the ionized gas contains ions and electrons in about equal numbers so that the resultant space charge is very small. We shall use the name plasma to describe this region containing balanced charges of ions and electrons.”
The ionization of the neutral particles is usually achieved through heating. As temperature rises, molecules become more energetic and transform in sequence from solid to liquid, gas, and a plasma state. In the plasma state, freely moving particles, including electrons and positively or negatively charged ions, make them electrically conductive and can attain electrical conductivities sometimes larger than metals such as gold and copper.
1.2 Plasma Generation in Nature and in the Laboratory
Plasmas comprise the majority of matter in the universe. Most of the stars are made of plasma. The space between the stars is filled with plasma, although at a much lower density than that inside the stars. On Earth, however, naturally occurring plasma is somewhat rare. In Earth’s atmosphere, the best-known plasma phenomenon is lightning. An average lightning bolt carries an electric current of about 100 kA and an approximate power output of 1 MW per meter, which rapidly heats the air in its immediate vicinity to a temperature of over 10,000°C. The sudden heating effect and the expansion of heated air give rise to a supersonic shock wave in the surrounding clear air. Once this shock wave decays to an acoustic wave, it is heard as thunder.
FIGURE 1.1
Aurora borealis as seen from International Space Station. (Courtesy of NASA.)
At an altitude of approximately 100 km, the atmosphere is conductive due to the ionization of neutral molecules by solar radiation, making this region of the atmosphere in a plasma state called the ionosphere. Long-distance communication is largely made possible by the presence of the ionosphere through the reflection of radio waves by the ionized layer. Aurora is another example of natural plasma on Earth (see Figure 1.1). At near-space altitudes, Earth’s magnetic field interacts with charged particles from the Sun. These particles are diverted and often trapped by the magnetic field. These trapped particles are most dense near the poles, causing ionizations of neutral particles in the atmosphere and thus accounting for the light emission of the aurora.
Although the presence of natural plasma on Earth is relatively scarce (Figure 1.2), the number of industrial applications of plasma technologies is extensive. Historically, the study of vacuum tubes and so-called cathode rays laid the initial foundation of much of our understanding of plasma, which led to the development of plasma lighting technologies since the 19th century. More energy-efficient fluorescent lamps have been available on the market for the past few decades. In recent years, high-output radio-frequency (RF)-powered lamps have been developed as a viable alternative to LED (light-emitting diode) lamps, whose manufacturing process also heavily relies on plasma technologies.
Another important application of plasma resides in the semiconductor manufacturing industry. The microelectronics industry would virtually be impossible without plasma since most processes in semiconductor device fabrication, including dry etching, deposition, and implantation, cannot be achieved by any other commercial method but plasma.
FIGURE 1.2
Solar plasma. Emission in spectral lines shows the upper chromosphere at a temperature of about 60,000 K. (Courtesy of NASA.)
Plasma is widely employed in the coating industry, in which its large enthalpy content, high temperature, and high deposition rates are advantageous for increased throughputs. Various materials, including plastics, complex alloys, composites, and ceramics, can be deposited over a large area in different shapes. In the plasma-spraying process (see Figure 1.3), the material to be deposited—typically in a powder form—is introduced into a plasma jet with a temperature on the order of 10,000 K. The material is melted and accelerated toward the surface of the substrate, where the molten droplets rapidly solidify and form the deposition layer.
Surface property modifications for different polymer materials are usually performed using plasma. Many common polymer surfaces are chemically inert and therefore pose challenges for use as substrates for applied layers. The modification of polymer surfaces by plasma treatment can improve surface characteristics such as adhesion promotion, enhancement of wettability and spreading, improved biocompatibility, functionalized surface, reduced surface friction, and tackiness. These unique surface modifications that can be achieved using the plasma process result from the effects of the photons and active species in the plasma to react with surfaces in depths from several hundred angstroms to microns without influencing the bulk properties of the polymer base material.
FIGURE 1.3
Plasma thermal spray coating. (Courtesy of NASA.)
Low-temperature, nonequilibrium plasmas are an emerging technology for abating volatile organic compound (VOC) emissions and other industrial exhausts, which have become an important environmental concern as most of them are carcinogens and harmful to living organisms. Abatement of these polluting substances is conventionally handled by water scrubbers or adsorbent filters to convert them to harmless products. However, for the abatement of diluted VOCs with low concentrations (<100 ppm), these conventional techniques are not suitable, mainly due to high-energy consumption. Among the alternatives, nonequilibrium atmospheric pressure plasma processes have been shown to be effective in treating a wide range of emissions, including aliphatic hydrocarbons, chlorofluorocarbons, methyl cyanide, phosgene, as well as sulfur and nitrogen oxides. The reduction of the power consumption relies on the selective production of reactive species like ions, radicals, and activated molecules by the plasma process without heating of the bulk volume.
1.3 Needs for Plasma Water Treatment
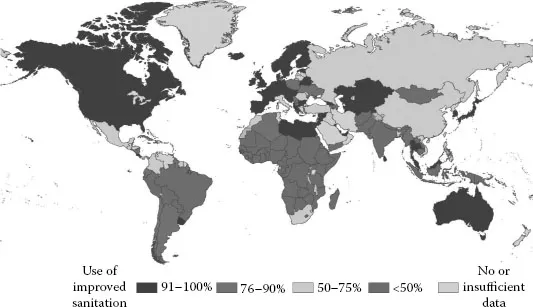
The availability of clean water is an issue that has paralleled the continual increase in water consumption due to both global population growth and the economic development in a number of developing countries. From a global perspective, an estimated 2.6 billion people are unable to acquire clean, safe drinking water (World Water Assessment Programme, 2009). The global picture shows great disparities between regions (Figure 1.4). Virtually the entire population of the developed regions uses improved facilities for water supply and discharge, but in developing regions only around half the population use improved sanitation facilities.
FIGURE 1.4
Use of sanitary water in rural areas, 2008. (From World Health Organization. (2010) Progress on sanitation and drinking-water.)
Contaminated water can be attributed to a number of factors, including chemical fouling, inadequate treatment, and a deficient or failing water treatment and distribution system. An additional important cause of the contamination is the presence of untreated bacteria and viruses within the water. The United Nations World Health Organization (WHO; 2010) estimated that nearly 35% of all deaths in developing countries were related directly to contaminated water. In the United States, the increased presence of Escherichia coli (E. coli) along with various other bacteria within some areas has also become a cause for national concern. In 2006, there was an outbreak of disease caused by E. coli found in spinach in 25 U.S. states, which caused thousands of illnesses and three deaths (U.S. Environmental Protection Agency Office of Water, 2009). In 2010, more than 500 million eggs were recalled after dangerous levels of Salmonella were detected. Salmonella may be caused by groundwater that has been contaminated by animal feces.
In an effort to inactivate these bacteria, traditional chemical treatments, ultraviolet (UV) radiation, and ozone injection units have been implemented for potable water delivery systems. The experimental success and commercialization of these water treatment methods are not, however, without deficiencies. With regard to human consumption of water, chemical treatments such as chlorination can render potable water toxic. Both UV radiation and ozone injection have been proven to be practical methods of bacterial inactivation in water, but the effectiveness of such methods largely depends on adherence to regimented maintenance schedules.
Plasma methods that effectively combine the contribution of UV radiation, active chemicals, and high electric fields have been considered as an alternative to these conventional water treatment methods (Locke, Sato, et al., 2006; Fridman, Gutsol, and Cho, 2007; Muhammad, 2010). Before considering direct application of plasma to water treatment (which is a major goal of this book), we discuss briefly the independent application of UV radiation, active chemicals, and high electric fields for the deactivation of microorganisms in water.
1.4 Conventional Water Treatment Technologies
Currently, there are many available methods of water treatment and decontamination, including chlorination, ozonation, UV radiation, in-line filters, and pulsed electric fields. Many of these systems are utilized in large industrial applications. However, methods such as chlorination, in-line filtering, and UV radiation are also applied in point-of-use applications, including treatment of swimming pool and well water. These methods have distinct advantages and disadvantages and are carefully analyzed next.
1.4.1 Chlorination
The technique of purification of water using chlorine was first proposed in the early 1800s. For the past 200 years, chlorine has remained both an acceptable and a widely employed method of treatment with regard to water disinfection due to its ease of use and associated efficiency for the inactivation of microorganisms. Regardless of the system size, it is one of the least-expensive disinfection methods. However, the chlorination of public drinking water supplies is meeting with strong resistance as people are more concerned about the health effects of the process as the toxicity of chlorine requires strict adherence to accepted concentration levels. An excess of chlorine in a drinking water supply could render the water toxic with regard to human ingestion. Unwanted disinfection by-products (DBPs) resulting from the interaction of chlorine with other chemicals present in water can prove corrosive and deteriorative to the system. Under some circumstances, chlorine can react with organic compounds found in the water supply to produce trihalomethanes (THMs) and haloacetic acids (HAAs) (Adams et al., 2005), both of which are highly carcinogenic. In addition, because a chlorination-based system must be continually replenished, the storage and transportation of this chemical becomes a significant hazard.
1.4.2 In-Line Filters
In-line filters are commonly used to remove undesirable substances from water. Many different types are commercially available, including activated filters, microfilters, and reverse osmosis filters. The key advantage to these filters is that they do not require power to operate, but there are two significant drawbacks to this method. Although these filters are capable of preventing microorganisms from passing through the system, they are incapable of inactivating them, resulting in bacterial growth in the filters. The small pores needed to trap microorganisms also inhibit the flow, resulting in pressure loss across the filter. Significant pressure losses in the system require a larger-size pump.
1.4.3 Pulsed Electric Field
The next method considered for inactivating microorganisms is pulsed electric field technology. Since the electric field associated with this method is not strong enough (membrane potential of more than 1 V can kill a bacterium) to initiate electrical breakdown in water, there is no resulting electric discharge. The deactivation of microorganisms is believed to be due to electroporation, a process that is the creation of holes in cell membranes, indicating that plasma-originated electric fields (for example, those in DBD streamers) might be sufficient. At nominal conditions, the energy expense for a two-log reduction is approximately 30,000 J/L (Katsuki et al., 2002). Researchers at the Technical University of Hamburg, Germany, reported pulsed electric field effects on suspensions of bacteria in water (Grahl and Markl, 1996). They reported that the external electric pulse produced a membrane potential of more than 1 V for the effective killing of bacteria.
1.4.4 Ultraviolet Radiation
Ultraviolet radiation generated by plasma has proven effective in decontamination processes and is gaining popularity, particularly in Europe, because it does not leave undesirable by-products in water. Measurement of this radiation is considered in terms of dosage, which is given as the product of intensity (watts per square centimeter, W/cm2) and contact time (seconds, s). Most bacteria and viruses require relatively low UV dosages for inactivation, which is usually in a range of 2,000–6,000 mW·s/cm2 for a 90% kill rate. For example, E. coli requires a dosage of 3,000 mW·s/cm2 for a 90% reduction (Wolfe, 1990). Cryptosporidium, which shows an extreme resistance to chlorine, requires a UV dosage greater than 82,000 mW·s/cm2. The criteria for the acceptability of UV disinfecting units include a minimum dosage of 16,000 mW·s/cm2 and a maximum water penetration depth of approximately 7.5 cm (Wolfe, 1990).
UV radiation in the wavelength range from 240 to 280 nm causes irreparable damage to the nucleic acid of microorganisms. The most potent wavelength of UV radiation for DNA damage is approximately 260 nm. Currently, there are two types of commercial UV lamps: low-pressure and medium-pressure mercury lamps. It is worth noting that the UV radiation from both types is generated in plasma. The former possesses a relatively low temperature and produces a narrow band of UV light with a peak near the 254-nm wavelength, whereas the latter produces a higher temperature and a broader band of UV and has a much greater treatment capacity, approximately 25 times higher than the former (Wolfe, 1990). The life of a UV lamp is relatively short, approximately 8,000–10,000 h, compromised by several additional factors, including biological shielding and chemical or biological film buildup on the surface of the lamp. An advantage of this system is that both the temperature and the pH of the treated water are not significantly affected, and no undesirable by-products are created (Wolfe, 1990). However, the total energy cost of the UV water treatment is high, similar to that for pulsed electric fields.
The UV photons can have two possible effects on a microorganism. One effect is through direct collisions with contaminants, causing the mutation of bacterial DNA. This prevents proper cellular reproduction and thus effectively inactivates the microorganism. Alternatively, the photons can provide the necessary energy to ionize or dissociate water molecules, thus generating active ch...