
eBook - ePub
Natural Antioxidants
Applications in Foods of Animal Origin
This is a test
- 414 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Natural Antioxidants
Applications in Foods of Animal Origin
Book details
Book preview
Table of contents
Citations
About This Book
In the recent years, considerable research has been carried out evaluating natural substances as antioxidative additives in food products, leading to novel combinations of antioxidants and the development of novel food products. In addition to their antioxidative capacity, these natural additives have positive effects on the human body with documented health benefits. This valuable new book provides an overview of natural antioxidants, their sources, methods of extraction, regulatory aspects, and application techniques, specifically focusing on different foods of animal origin to improve their oxidative stability.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Natural Antioxidants by Rituparna Banerjee, Arun K. Verma, Mohammed Wasim Siddiqui in PDF and/or ePUB format, as well as other popular books in Medicina & Nutrición, dietética y bariatría. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
MECHANISM OF OXIDATION IN FOODS OF ANIMAL ORIGIN
Food Technology and Innovation Center of Excellence, Department of Agro-Industry, School of Agricultural Technology, Walailak University, Thasala 80160, Nakhon Si Thammarat, Thailand
* Corresponding author. E-mail: [email protected]
CONTENTS
Abstract
1.1 Introduction
1.2 Impact of Lipid Oxidation in Foods of Animal Origin
1.3 Basic Mechanism of Lipid Oxidation in Foods of Animal Origin
1.4 Effect of Intrinsic Factors and Processing Parameters in Lipid Oxidation in Foods of Animal Origin
1.5 Myoglobin Oxidation in Foods of Animal Origin
1.6 Interrelationship Between Lipid Oxidation and Myoglobin Oxidation in Foods of Animal Origin
1.7 Interaction Between Lipid Oxidation Products and Myoglobin
1.8 Conclusion
Keywords
References
ABSTRACT
Lipid and myoglobin oxidations significantly impair the quality of foods of animal origin because these reactions deteriorate flavor and color, induce the loss of nutritional value and cause technological problems during processing. Lipid and myoglobin oxidations are coupled and such reactions can occur via non-enzymatic and enzymatic routes. Several factors have been reported to enhance the oxidation of lipid in muscle foods including species, muscle type, fatty acid composition, endogenous antioxidants (AH), temperature, metal ions, sodium chloride, muscle pH, and processing parameters. It is most likely that the prooxidant effect of heme proteins, especially myoglobin, is a prime factor influencing the lipid oxidation in muscle foods. On the other hand, lipid oxidation results in a wide range of aldehyde products, which can cause the oxidation of myoglobin. The interaction between myoglobin and aldehydic lipid oxidation products can alter myoglobin redox stability and finally results in muscle discoloration. As a consequence, the oxidation of both lipid and myoglobin directly affect the quality and acceptability of muscle foods and those reactions seems to promote each other.
1.1 INTRODUCTION
The problems associated with oxidation in foods of animal origin, particularly meat and muscle foods, have gained much interest as they relate to flavor deterioration, discoloration, loss of nutritional value and safety, biological damage, ageing, and functional property changes. Meat and other muscle foods are complex foods with highly structured nutritional compositions (Rodriguez-Estrada et al., 1997). Muscles are composed of water, proteins, lipids, carbohydrates, vitamins, and minerals in variable amounts depending on several factors such as breeds, muscle types, dietary, and growth performance (Wattanachant et al., 2005). Oxidation is a major cause of quality deterioration for a variety of raw and processed muscle foods during handling, processing, and storage. Lipid, protein, pigment, and vitamin in muscle tissue are susceptible to oxidative reactions. These changes resulted from reactions of active oxygen species, free radicals, enzymes, and prooxidants with unsaturated fatty acids in lipids, amino acids in proteins, heme groups in pigments and the chains in vitamins with conjugated double bonds. However, lipid oxidation and the oxidation of heme proteins, particularly myoglobin, in muscle foods are major deteriorative reactions which occur in a concurrent manner and each process appears to enhance the other. During oxidation of Mechanism of Oxidation in Foods of Animal Origin 3 oxymyoglobin, both superoxide anion and hydrogen peroxide are produced and further react with iron to produce hydroxyl radical. The hydroxyl radical has the ability to penetrate into the hydrophobic lipid region and hence facilitates lipid oxidation. The prooxidant effect of heme proteins on lipid oxidation is concentration-dependent. At equimolar concentrations, oxymyoglobin shows higher prooxidative activity toward lipid than metmyoglobin. However, the catalytic activity of metmyoglobin is promoted by hydrogen peroxide. The reaction between hydrogen peroxide and metmyoglobin results in the formation of two active hypervalent species, perferrylmyoglobin and ferrylmyoglobin, which are responsible for lipid oxidation. Additionally, lipid oxidation results in a wide range of aldehyde products, which are reported to induce the oxidation of oxymyoglobin. Studies in muscle foods have been focused mainly the interaction between myoglobin and aldehydic lipid oxidation products. Metmyoglobin formation is generally greater in the presence of unsaturated aldehydes than their saturated counterparts of equivalent carbon chain length. In addition, increasing chain length of aldehydes, from hexenal through nonenal, results in the increased metmyoglobin formation. Moreover, aldehydes alter myoglobin redox stability by increasing oxymyoglobin oxidation, decreasing the metmyoglobin reduction via enzymatic process, and enhance the prooxidant activity of metmyoglobin (Chaijan, 2008). Therefore, the oxidation of both lipid and heme proteins directly affect the quality and acceptability of muscle foods and the lowering of such a phenomenon can enhance the shelf-life stability of those foods. To design strategies to inhibit the progression of oxidative reactions in foods and biological systems, it is important to understand the nature of these reactions and how they are influenced by both intrinsic and extrinsic factors. This goal may be achieved through a better understanding of the reaction kinetics including the rate at which the reaction takes place, the effective factors (mainly temperature, concentration of reactants and products, and presence of catalysts), and how these two are related. This chapter deals with the mechanism of oxidation, especially lipid and myoglobin, in foods of animal origin emphasizing on meat and muscle foods. The interaction between lipid and myoglobin oxidations and the effect of various food-processing applications on lipid and myoglobin oxidations are also discussed.
1.2 IMPACT OF LIPID OXIDATION IN FOODS OF ANIMAL ORIGIN
Lipid oxidation is one of the important reactions in food and biological systems because it has deleterious effects on polyunsaturated fatty acids (PUFA) and other lipid substrates, causing significant losses in food quality, health, and well-being (Chaijan et al., 2006; Chaijan, 2008). Lipid oxidation in food systems is a detrimental process. It is difficult to find a food component that would not be capable of affecting lipid oxidation because lipids are only a part of a food product (Kolakowska, 2002). Generally, lipid oxidation deteriorates the sensory quality and nutritive value of a product, poses a health hazard, and presents a number of analytical problems (Kolakowska, 2002). Lipid oxidation is also one of the main factors limiting the quality and acceptability of meats and other muscle foods, especially following refrigerated and frozen storages (Zamora & Hidago, 2001; Renerre, 2000; Morrissey et al., 1998). Oxidation of lipids is accentuated in the immediate post-slaughter period, during handling, processing, storage, and cooking. This process leads to discoloration, drip losses, off-odor and off-flavor development, texture defects, and the production of potentially toxic compounds in meat products (Richards et al., 2002; Morrissey et al., 1998).
Hydroperoxide is a primary oxidation product during storage of foods which is readily decomposed to a variety of volatile compounds including aldehydes, ketones, and alcohols (Frankel et al., 1984). The formation of the secondary lipid oxidation products is one of the main causes of the development of undesirable odors in muscle foods. Human olfactory receptors usually have remarkably low organoleptic thresholds to most of these volatile compounds (Ke et al., 1975; McGill et al., 1977). The effect of lipid oxidation and off-odor development in postmortem fish has been reported. Lipid oxidation is mainly associated with the rejection by consumer due to the off-odor and off-flavor. Flavor is a very complex attribute of meat palatability. Rancid or fishy odor has been identified as a common off-flavor associated with fish flesh and directly related with the formation of the secondary lipid oxidation products (Ke et al., 1975; McGill et al., 1977; Sohn et al., 2005; Thiansilakul et al., 2010). Varlet et al. (2006) reported that carbonyl compounds, such as heptanal or (E,Z)-2,6-nonadienal, show a high detection frequency and odorant intensity in salmon (Salmo salar), giving the flesh its typical fishy odor. The fishy volatiles identified in the boiled sardine were dimethyl sulfide, acetaldehyde, propionaldehyde, butyraldehyde, 2-ethyl-furan, valeraldehyde, 2,3-pentanedione, hexanal, and 1-penten-3-ol (Kasahara & Osawa, 1998).
Lipid oxidation usually causes a decrease in consumer acceptance. However, in some cases, lipid oxidation leads to enhancement of product quality such as the enzymatic production of fresh fish aromas and the cured meat flavor derived from lipid oxidation during ripening (Ladikos & Lougovois, 1990). A notable exception is observed in dry cured country hams and some fermented sausages and the desirable flavor of which does not occur until hydrolysis of some of the fat and a certain degree of oxidation has taken place during ripening (Pearson et al., 1977). On the other hand, lipid oxidation during cooking may be a source of intermediate which react with other components to give important constituents of the desirable flavor of normal cooked meat (Enser, 1987). The types of flavor developed from the volatile lipid oxidation compounds depend on a multitude of complex interactions, concentration ranges, and the medium in which they are tasted (Frankel, 1984). Many of the reactions involved in the formation of volatile aroma compounds from lipid, follow the same basic pathways for both thermal and rancid oxidation and similar volatile products are formed. However, subtle differences in the precise mechanisms of oxidation under storage conditions and under thermal processing lead to mixtures of volatiles exhibiting both qualitative and quantitative differences (Mottram, 1987).
1.3 BASIC MECHANISM OF LIPID OXIDATION IN FOODS OF ANIMAL ORIGIN
The lipid oxidation in foods of animal origin is assumed to proceed along a free radical route (autoxidation), photooxidation route and enzymatic route. The oxidation mechanism is basically explained by invoking free-radical reactions, while the photooxidation and lipoxygenase (LOX) routes differ from it at the initiation stage only. For this reason, they can be treated as different forms of free radical reaction initiation.
1.3.1 FREE RADICAL OXIDATION
The two major components involved in lipid oxidation are unsaturated fatty acids and oxygen. In this process, atmospheric oxygen is added to some fatty acids, producing unstable intermediates that finally breakdown to form unpleasant flavor and aroma compounds (Erickson, 2003). Although enzymatic and photogenic oxidation may play a role, the most common and important process by which unsaturated fatty acids and oxygen interact is a free radical mechanism (Erickson, 2003). A free radical reaction or autoxidation is the main reaction involved in oxidative deterioration of food lipids, including foods of animal origin (Hoac et al., 2006). It is a chain reaction that consists of initiation, propagation, and termination reactions, and involves the production of free radicals (Gunstone & Norris, 1983; Nawar, 1996; Renerre, 2000; Fig. 1.1). Oxidation is initiated by radicals present in living organisms (e.g., hydroperoxide, hydroxide, peroxide, alcoxy, and alkyl) or by thermal or photochemical homolytic cleavage of an R-H bond. The oxidation activation energy and reaction rate at this stage depend on the type of initiator and the number of unsaturated bonds in the substrate. The dissociation energy of the C-H bonds in saturated fatty acid depend on the length of the fatty acid carbon chain and is similar in fatty acid, their esters and in triacylglycerols (Litwinienko et al., 1999). In unsaturated acids, the weakest C-H bond is found in the bis-allylic position. The activation energies are 75, 88, and 100 kcal/mol for bis-allylic, allylic, and methylene hydrogens, respectively (Simic et al., 1992). A three-step simplified free-radical scheme has been postulated as follows:
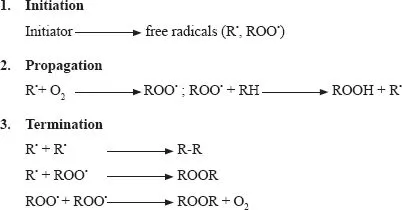
FIGURE 1.1 Mechanism of lipid oxidation via free radical route.
Initiation occurs as hydrogen is abstracted from an unsaturated fatty acid, resulting in a lipid-free radical, which, in turn, reacts with molecular oxygen to form a lipid peroxyl radical. Initiation is frequently attributed in most foods, including muscle foods, to react ion of the fatty aci...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- List of Contributors
- List of Abbreviations
- Preface
- 1. Mechanism of Oxidation in Foods of Animal Origin
- 2. Natural Antioxidants: Occurrence and Their Role in Food Preservation
- 3. Potential Applications of Natural Antioxidants in Meat and Meat Products
- 4. Natural Antioxidants: Control of Oxidation in Fish and Fish Products
- 5. Natural Antioxidants in Poultry Products
- 6. Methods and Their Applications for Measuring and Managing Lipid Oxidation: Meat, Poultry, and Seafood Products
- 7. Application of Natural Antioxidants in Dairy Foods
- 8. Antioxidant Dietary Fiber: An Approach to Develop Healthy and Stable Meat Products
- 9. Control of Lipid Oxidation in Muscle Food by Active Packaging Technology
- Index