
Chemistry and Technology of Plant Substances
Chemical and Biochemical Aspects
- 358 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Chemistry and Technology of Plant Substances
Chemical and Biochemical Aspects
About This Book
Chemistry and Technology of Plant Substances: Chemical and Biochemical Aspects demonstrates the progress and promise of developing new chemical substances from renewable sources of chemical raw materials. The volume brings together new achievements in the field of research and processing of plant raw materials and the synthesis of natural compounds for the production of biologically active substances and drugs. The volume looks closely at the rational use of renewable raw materials, which is the source of new compounds and intermediates for the chemical industry. It covers a wide range of problems associated with the use of the components of plants to produce new substances with a wide variety of purposes.
According to the latest estimates, plants form about a million chemical substances. In some cases, plant products have pharmacological or biological activity that can be of therapeutic benefit in treating diseases. In addition, due to the structural diversity of plant material, chemical synthesis is easily reachable. Synthetic analogs of natural products with improved potency and safety can be prepared by chemical synthesis. Such synthetic analogs are safer for humans. Plant materials are often used as starting points for drug discovery. Chemistry and Technology of Plant Substances: Chemical and Biochemical Aspects presents the theoretical trends and recent practical achievements on complex processing of plant-based raw materials. Low molecular weight components, isolated from plant material, are widely used in fine organic synthesis. High molecular weight polysaccharides of conifers and other greens, such as pectin and hemicellulose, are the basis for the creation of anticoagulants and other drugs. The range of research papers presented in the book is quite wide: from fundamental and applied problems of wood chemistry and organic synthesis to biological activity of natural compounds.
The book provides valuable information for those skilled in organic chemistry, chemical engineers, researchers and scientists as well as for faculty and upper-level students.
This volume, Chemistry and Technology of Plant Substances: Chemical and Biochemical Aspects, was created on the initiative of Emanuel Institute of Biochemical Physics of the Russian Academy of Sciences (Moscow) and the Institute of Chemistry of Komi Scientific Center of Ural Branch of the Russian Academy of Sciences (Syktyvkar).
Frequently asked questions
Information
PART I
Components of Plant Origins: Synthesis, Modification, and Properties
CHAPTER 1
SYNTHESIS AND TRANSFORMATIONS OF 2,3-SECOTRITERPENE DERIVATIVES OF BETULIN
CONTENTS
ABSTRACT
1.1 INTRODUCTION
1.2 SYNTHESIS OF THE BASIC 2,3-SECOTRITERPENOIDS
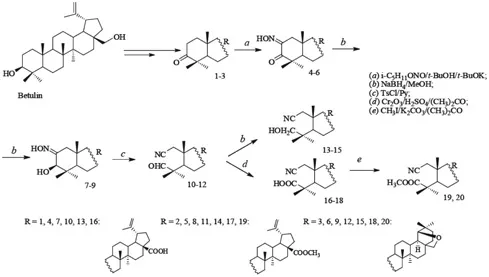
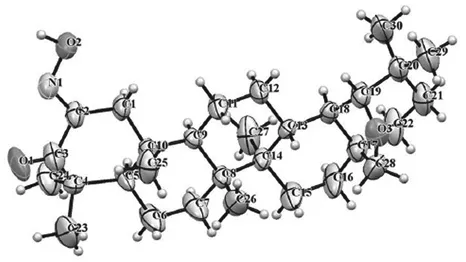
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- ABOUT THE EDITORS
- Table of Contents
- List of Contributors
- List of Abbreviations
- From Fundamental Science to New Technologies
- Preface
- Introduction
- Part I: Components of Plant Origins: Synthesis, Modification, and Properties
- Part II: Biological Activity of Plant Substances
- Glossary
- Index