
- 273 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Microcompartmentation
About this book
Microcompartmentation refers to nonhomogeneous distribution of solutes in compartments of cells or associated structures without intervening membranal barriers. Such variations in concentration of ions, metabolites, intracellular messengers, and nutrients can introduce significant heterogeneity in subcellular function and regulation. The current focus on biological examples of microcompartmentation provides ample evidence for its importance and the role of physical structure in determining local chemical environments. The examples present in the different chapters include microcompartmentation of Ca2+,H+,ATP. ADP, O2, glycolytic intermediates, fatty acids, amino acids, and nucleic acid precursors. In reviewing these systems, the authors provide a useful resource for experimental approaches to study microcompartmentation and provide the basis for future studies of its role in regulation of cell functions.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
On the Internal Environment of Animal Cells
Tables of Contents
I. Introduction
II. Thin Section Electron Microscopy and Cell Fractionation
III. Are the Aqueous Compartments Concentrated Solutions?
A. The Devaux Effect
B. Cell Stratification
C. The Reference Phase Technique
D. Section Summary
IV. High Voltage Electron Microscopy
V. Water and Micromolecules in the Aqueous Compartments
A. Micromolecules
B. Intracellular Water.
C. Intracellular Interfaces
1. Surface Area
2. The Influence of Surfaces on Adjacent Water.
3. Significance to Compartmentation
VI. On the Concept of “Levels of Biological Organization”
Acknowledgments
References
I. Introduction
Compartmentation is one of the most pervasive features of biological systems,1 so it should come as no surprise to observe it within cells. A question much more difficult to answer, and therefore more interesting, concerns the extent to which it occurs. Friedrich1 has referred to “microcompartmentation” as metabolite sequestration by coupled enzymes in which the dimensions of the compartment take on those of the metabolite plus surrounding boundaries of the participating enzymes. The other authors in this book will, no doubt, offer their own views of that term, but I will use it as given above because it allows some interesting consequences to emerge, transcending the metabolic context within which the phenomenon is usually discussed.
That microcompartmentation exists is beyond doubt, and the abundant evidence for it will unfold in this book, as it has in others.2-5 However, we should ask, how widespread is its occurrence within the cell? It seems worthwhile to consider that question against a backdrop of the broader question of the nature of intracellular organization. That is my major task in this chapter. Because the approach taken here will be more global than the usual detailed description of the bits and pieces of cells, it may take on a vitalistic air which, however, I hope to avoid by select choice of good experimental evidence. Therefore, what follows should in no way be considered to be a “review” of the literature. In fact, it comes closer to being a review of reviews.
I will begin by considering results obtained from two powerful and very widely used techniques, confessing at the outset that I think we may have been deceived by what they have revealed.
II. Thin Section Electron Microscopy and Cell Fractionation
What has the use of these methods told us about the eukaryotic cell? Figure 1 shows a typical result obtained from thin section electron microscopy (TS-EM). Many of us were raised on these images, and they are so familiar that they stir little interest per se. Moreover, it is clear that TS-EM has been invaluable in our attempts to understand cell structure, but consider that they reveal only about 25% of the mass and volume that actually exist in an intact living cell. Understandably, cell biology has concentrated on what can be seen, and that has been profitable, but is it not also important to ask about the remainder – the vast majority? What is it actually like in all those "empty spaces" that are not revealed by TSEM? For convenience, we can refer to the latter as reflections of the existence of what I will call the aqueous compartments, the "soluble phases" of the nucleus, cytoplasmic membrane-bounded organelles, and the intervening aqueous cytoplasmic space, often called "cytosol", a term with several meanings.5 It is widely appreciated that TS-EM tells us nothing about these compartments, since they are washed away during the preparative procedures. Thus, we will stop here for the moment and turn to cell fractionation.
Table 1 illustrates what happens typically when cells are disrupted in a "suitable" buffer. These particular data are taken from the work of Lahav et al.,6 but literally thousands of similar studies would reveal a similar outcome; a very large proportion of the cell’s proteins (and other macromolecules) are released, remaining in solution when the homogenate is centrifuged at very high speeds. This result has led to the tempting possibility that these materials were also soluble when in the intact cell. Indeed, many seem to have yielded to temptation: that interpretation has, with some caution at times, been widely applied. While few would argue that cell fractionation provides quantitative, unambiguous evidence for the actual intracellular location of a given molecule, I believe it is fair to say that the "soluble phases" from cell fractionation are commonly perceived to be an approximation of reality. Setting that dubious assumption aside for the moment, let us return to the images from TSEM (Figure 1).
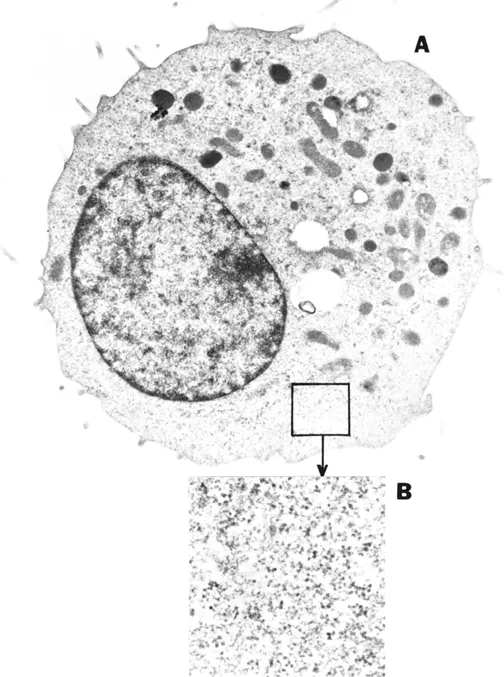
Figure 1. Transmission electron photomicrograph of a thin section of a mouse L-929 cell (A) at an original magnification of × 13,500. An enlarged area of the cytoplasm (original magnification × 48,500) is shown in (B). Details of preparation are given in reference 30. Provided by Murali Pillai.
It is easy to understand how the "blank spaces" in the images from TS-EM have been translated into the "soluble phases" of cell fractionation, and vice versa. That interpretation generates the impression that a relatively sharp boundary exists between the ultrastructure we can see, and the surrounding "structureless solution", which is presumably composed of a crowded collection of macromolecules, metabolites, inorganicions, and, of course, water. Moreover, much of the metabolic activity of cells is assigned to that location, since the soluble phase contains many enzymes. The question of prime importance is whether evidence from methods other than TS-EM and cell fractionation support that generally accepted paradigm.
Table 1 Protein and DNA Contents of Subcellular Fractions of Rat Liver Cellsa
| Total homogenate (%) | ||
| Fraction | Protein | DNA |
| Nuclei | 12 | 91 |
| Mitochondria | 14 | 6 |
| Mixed (2,4) | 8 | 4 |
| Microsomes | 15 | 0 |
| Soluble | 56 | 0 |
a Data are from Lahav et al.,6 who provide details of the fractionation procedure.
In the remainder of this article I will refer to evidence from a variety of sources and cell-types that should, in my view, compel us to discard that widely held conception of the organization and function of the eukaryotic cell. I believe that impression is not only incorrect, but also misleading, because it deludes us into believing that we know something about cells that we actually do not. Having accepted the burden of proof, it now becomes necessary to sample the evidence, and "sample" is the correct term.
III. Are the Aqueous Compartments Concentrated Solutions?
I believe the answer is no, and we have had reason to suspect that for at least half a century. Let us examine one early example, the work done by Chambers and Kopac in the late 1930s and reviewed by Chambers in 1940.7 They introduced oil droplets into echinoderm eggs and observed their behavior. Their 48-year-old results and interpretations are still revealing.
A. The Devaux Effect
Chambers and Kopac observed that droplets of various kinds of oil would spontaneously coalesce with the eggs, entering the cytoplasm. Alternatively, the droplets were injected, with similar results; if the cytoplasm was not injured, the oil drops remained perfectly spherical. However, if the cytoplasm was intentionally damaged, the droplet would then undergo a surface crinkling, known as the "Devaux effect". It was pointed out that this crinkling occurs when proteins in solution are absorbed at an oil-water interface at monolayer coverage concentrations.
Kopac and Chambers proposed reasonably that the absence of the Devaux effect in undamaged cells reflected the absence of significant concentrations of diffusible proteins of the size that would absorb onto the droplets. Moreover, Kopac devised a way to evaluate protein absorption on the droplets. That was accomplished by the "drop retraction" method in which an introduced droplet, retained by the injecting micropipette, was partially withdrawn back into the pipette. He reasoned that if some protein had been absorbed, but less than a monolayer, then the Devaux effect would, in principle, suddenly occur when the volume of the droplet was reduced to the critical level. Kopac calculated that an oil droplet 10 μm in diameter, of 3.14 × 10-6 cm-2 surface area, would require only about 3 × 10-6μg of pr...
Table of contents
- Cover
- Title
- Copyright
- PREFACE
- THE EDITOR
- CONTRIBUTORS
- TABLE OF CONTENTS
- Dedication
- Chapter 1: On the Internal Environment of Animal Cells
- Chapter 2: Functional Compartmentation of Carbohydrate Metabolism
- Chapter 3: Mitochondrial Distribution and O2 Gradients in Mammalian Cells
- Chapter 4: Involvement of Microcompartmentation in the Regulation of Cell Proliferation
- Chapter 5: Diffusion and Ultrastructural Adaptive Responses in Ectotherms
- Chapter 6: Microcompartmentation of Metabolite Transport in Mitochondria
- Chapter 7: The Membranes Involved in Proton-Mediated Free-Energy Transduction: Thermodynamic Implications of their Physical Structure
- Chapter 8: Microcompartmentation of DNA Precursors
- Chapter 9: Function of Ambiquitous Proteins in a Heterogeneous Medium
- Chapter 10: Microzonation of ATP and pH in the Aqueous Cytoplasm of Mammalian Cells
- Chapter 11: Hydrogen and Calcium Ion Diffusion in Axoplasm
- Chapter 12: Fluorescence Digital Imaging Microscopy — Spatial Distribution of Ca2+ and H+ in Single Cells
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Microcompartmentation by D.P. Jones in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biology. We have over one million books available in our catalogue for you to explore.