
- 167 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Complement Infectious Diseases
About this book
This monograph has been written with the notion that it may be read by complementologists relatively untrained in microbiology and infectious diseases and by microbiologists and infectious diseases specialists relatively untrained in complementology. Thus, abbreviations are used sparingly and jargon has hopefully been minimized. Furthermore, I have attempted to offer general background information where it seemed relevant or helpful.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
THE COMPLEMENT SYSTEM
I. The Complement System
The complement system is a group of plasma proteins that interact to produce a variety of physiological and pathological effects. The effect(s) produced in a given circumstance depends upon the substance activating the complement system, the step at which the system is activated, and the point at which activation terminates.
A general principle is that the various proteins or components of the system circulate in inactive form. One component upon activation may then activate the next component in the sequence or “cascade”. Activation may involve internal rearrangement or cleavage of the protein so as to expose functional, usually enzymatic, sites, or aggregation of components may produce an effective complex. Natural circulating inhibitors compete with substrate proteins for sites on components and overall function represents the balance among activators, substrates, and inhibitors.
Complement proteins are produced in vivo and in vitro by a variety of tissues and cell types, including hepatocytes,1 liver tissue,2,3 reticuloendothelial cells,4-7 and small intestinal epithelial cells.8,9 At least for the third component, though multiple cell types can be demonstrated in vitro to produce the protein, the hepatocyte appears to be the primary in vivo site of synthesis.10 Catabolism involves constant and relatively rapid decay of the proteins, perhaps 1 to 3% of the plasma pool per hour.11
A. Terminology
The complement literature bristles with complex nomenclature, which serves as a major barrier to wider understanding. Proteins of the system are listed in Table 1. Certain principles apply to general nomenclature. Complement is indicated by the capital letter “C” (the older use of “C” has been discarded). Components of the classical pathway and terminal sequence (see below) are indicated by “C” for complement followed by a number representing the order in which the component was discovered; thus, C1 was discovered first, then C2, etc. Regrettably, these components do not react in the precise order in which they were discovered. Terminology of the alternative pathway components (see below) remains unsettled, though custom and preliminary agreement have strongly favored use of the properdin system terminology.12 By this usage, proteins are called “factors” and distinguished by capital letters (e.g., factor B, factor D); properdin itself is called only “P”.
Activated components may be indicated by a bar above the letter (e.g., ). Alternatively, cleaved subunits of components may be indicated by lowercase letters (a, b, c, d, etc.); some of these subunits are active (e.g., C3b, C5a), others inactive degradation products (e.g., C3d). Inhibitors or inactivators may be designated by functional (e.g., C1 INH, C3b INA) or biochemical (β1H) names. Figure 1 illustrates these principles using C3 as an example.
B. C3
The third component of complement, C3, is present in serum in highest concentration, 1200 μg/df.11 Sometimes referred to as β1C in recognition of its migration on serum protein electrophoresis, this molecule occupies a central position in the complement system, both functionally and biochemically (Figure 2).
Table 1
COMPLEMENT PROTEINS
COMPLEMENT PROTEINS
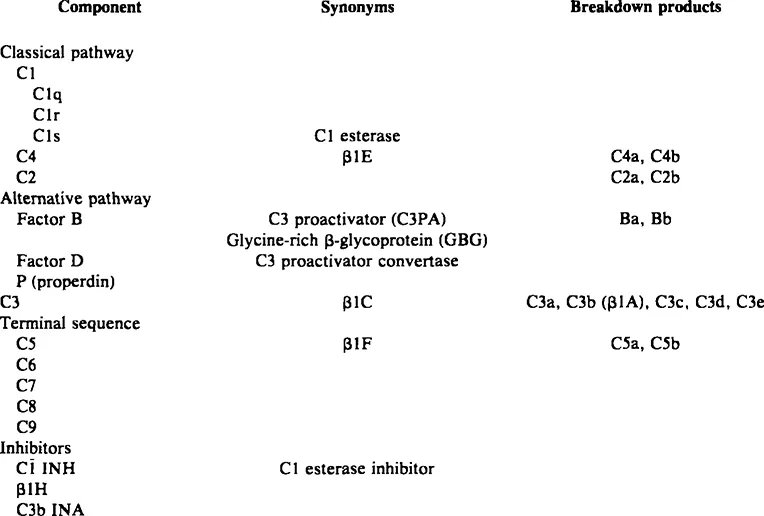
Activation of C3 (about 180,000 dalton) occurs upon cleavage into the two subunits C3a (about 10,000 dalton) and C3b (about 165,000 dalton) (Figure 1). Enzymes capable of cleaving C3 (C3 convertases) may be generated through the classical or the alternative pathway. The antigen upon which the convertase is generated becomes the site of deposition of nascent C3b particles; the “acceptor” sites do not appear to be specific “receptors” for C3b but rather nonspecific but stable sites to which C3b is bound by hydrophobic and by either ester or imidoester bonds.13
Many molecules of C3 may be activated by a single convertase.11 Some of these molecules will remain in fluid phase, where they are rapidly inactivated. Inactivation involves first the binding to C3b of β1H globulin, a normal serum protein, following which C3b inactivator (C3b INA) cleaves and degrades C3b.14,15 Law et al.15 suggested that C3b INA cleaves C3b to C3b’, a molecule that is antigenically intact but that has lost much of its functional activity. In subsequent degradation, proteolytic enzymes such as trypsin or plasmin cleave C3b’ to C3c and C3d.
Molecules of C3b that become bound to acceptor sites on activating antigens are presumably partly protected from the effects of β1H and C3b INA, so that they may mediate the biological effects of C3b. Activity of C3b depends therefore on the balance among the number of C3 convertase sites, continued activity of the C3 convertases (that have their own inhibitors), numbers of C3b molecules bound to “acceptor” sites, and relative protection of C3b from interaction with β1H. The amplification built into the complement system at several steps, including C3 activation, presumably represents an important part of this balance.
One important function of C3b is to initiate the terminal sequence of the complement system (see below). More important is that C3b may provide a ligand between the antigen to which it is bound and certain cells and tissues with specific receptors for C3b.16 These receptor-bearing cells include polymorphonuclear leukocytes,17 monocytes and macrophages,18,19 B lymphocytes,20 human erythrocytes,21 platelets from some species other than man,17 and renal glomeruli.22 The functions of these receptors and the C3b ligand will be discussed in subsequent sections and chapters.
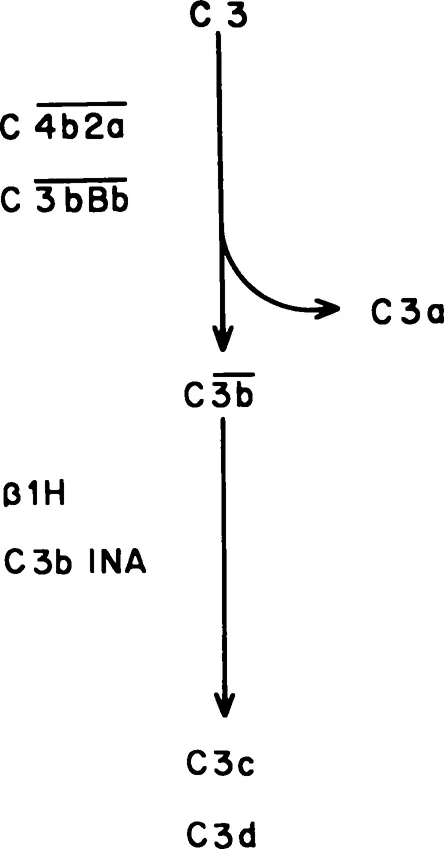
FIGURE 1. Principles of complement nomenclature as illustrated by activation-inactivation of C3. The native molecule (C3) can be cleaved by either the classical pathway or the alternative pathway C3 convertase (C4b2a or C3bBb, respectively). The smaller fragment (C3a) is soluble and rapidly inactivated. The larger fragment (C3b) may become bound to membranes or immune complexes; the fact that it is an active molecule can be indicated by the bar (). Natural inhibitors (β1H, C3b INA) inactivate the molecule, in this case by cleaving C3b into the inactive subunits C3c and C3d.
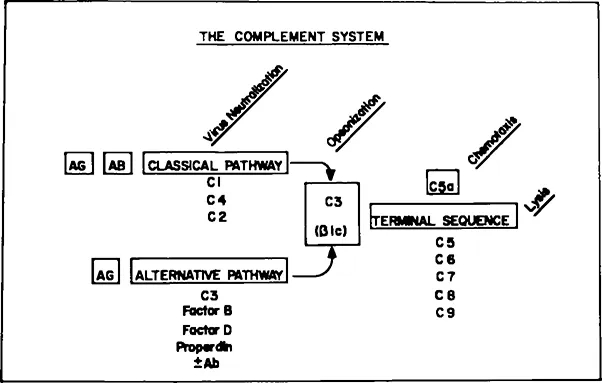
FIGURE 2. Schematic diagram of the complement system.
C. The Classical Pathway
Hemolysis of sheep erythrocytes, the standard model for the complement system, is based upon activation of the classical pathway. In this model, the Forssman antigen on the erythrocyte surface reacts specifically with rabbit antibodies, which then arm C1, the first component of complement. C1 is a trimolecular complex bound by calcium ions; the three subunits are designated C1q, C1r, and C1s. C1q binds to exposed sites of the Fc fragments of immunoglobulins G or M; internal rearrangement of C1 results in expression of the C1s-associated esterase (C1 esterase or ). The C1 esterase can then cleave C4 into its subunits C4a, which remains in fluid phase, and C4b, which remains membrane-bound. The combination of C1s and C4b cleaves C2. The smaller fragment, C2b, is dissipated; the larger fragment, C2a, becomes bound to the C4b molecule to form the classical pathway C3 convertase, i.e., the enzymatic activity, generated through the classical pathway by antigenantibody complexes, capable of cleaving C3.
For every immunoglobulin-C1q complex, there may be multiple membrane-bound C4b2a complexes, another example of the characteristic amplification of the complement system. The active CT (C1 esterase) can be inhibited by the esterase inhibitor. Inhibitors of C4b have been described.23 C2a is a highly unstable molecule, undergoing decay from the complex in a matter of minutes.11 Thus, even within the province of the classical pathway, one can see the balancing principles of amplification and inhibition.
While no definite physiological or pathological role has been identified for either C4a or C2b, the smaller fragments released upon activation of C4 and C2, they may have kinin-like properties of vasodilation.24 Otherwise, with the exception of potential roles in viral infections (see Chapter 5), the classical pathway proteins serve primarily intermediary functions through activation of C3 and the terminal sequence.
D. The Alternative Pathway
In 1954, Pillemer and colleagues12 described a system in human serum that appeared different from the classical hemolytic pathway. The distinctive protein of this system was properdin, which could react with a crude preparation of yeast cell walls (zymosan) to activate C3, bypassing C142. Later, they suggested that bacterial cell walls, endotoxin, and neutral polysaccharides could also activate this system,25 which had great potential importance to immunobiology. However, methods available then did not permit answers to stringent criticism of the data.26,27 Then, in the late 1960s and early 1970s, several lines of investigation established that there must be an alternative to the classical pathway. Guinea pigs deficient in C4 were healthy a...
Table of contents
- Cover
- Title Page
- Copyright Page
- Table of Contents
- Chapter 1 The Complement System
- Chapter 2 Human Complement Deficiencies
- Chapter 3 Bacteria
- Chapter 4 Fungi
- Chapter 5 Viruses
- Chapter 6 Parasites
- Chapter 7 Complement and Infectious Diseases
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Complement Infectious Diseases by Douglas P. Fine in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biology. We have over one million books available in our catalogue for you to explore.