
eBook - ePub
Handling of Radiation Accident Patients
by Paramedical and Hospital Personnel Second Edition
This is a test
- 228 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handling of Radiation Accident Patients
by Paramedical and Hospital Personnel Second Edition
Book details
Book preview
Table of contents
Citations
About This Book
This book provides guidelines, procedures, and techniques for emergency support personnel involved with handling radiation accident patients. Prepared by a former emergency medical responder, this book amplifies the level of radiological response training provided to emergency medical technicians and emergency room physicians and nurses. Supporting graphics, references, and a glossary help readers understand the critical aspects of emergency trauma treatment.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Handling of Radiation Accident Patients by Thomas A. Carder in PDF and/or ePUB format, as well as other popular books in Law & Forensic Science. We have over one million books available in our catalogue for you to explore.
Chapter 1
BASIC RADIOLOGICAL SCIENCE
PREVIEW
NUCLEAR RADIATION!!?? What is it!!?? Will it make you glow at night!!?? Will it make you invisible!!?? Will it turn you into a zombie!!??
NO
The images of science fiction and dramatic sensationalism have affected the minds of rational people. In this Chapter, you should be able to decide for yourself just what is nuclear radiation. Topics include:
- The composition of matter
- An example of how nuclear radiation can be produced
- How nuclear radiation may cause damage to living tissue
- A comparison of the relative penetration of nuclear radiation into matter
- Non-nuclear radiation
- What happens when nuclear radiation is stopped
This Chapter is the most "technical" of all and may seem to provide the least practical information about handling of radiation accident patients. However, those who have a basic understanding of the nature and properties of nuclear radiation and radioactive material appear to have the best mixture of confidence and respect- confidence from knowing the boundaries of the possible hazards of radiation and radioactivity, but enough respect to prevent hotdog heroism. Therefore, I will present what I know about the "physics" of radiation and radioactive material.
Section 1 Introduction to Radiological Science
As stated in the Preview of this Chapter, nuclear radiation cannot make anyone glow at night. Radioactive materials such as radium on luminous, glow-in-the-dark watch and clock dials might glow, but not people. Although some may want to be invisible, nuclear radiation cannot make you so. It is not possible for nuclear radiation to turn you into something that does not exist, i.e., a zombie.
Radiation comes in many types. Webster defines radiation as "to proceed in divergent lines from any central point or surface." Examples of radiation include:
- Heat
- Visible, Infrared, and Ultraviolet light
- Microwave
- Radio and Television signals
- X-ray
- Nuclear
So, "radiation" is not so bad after all. Our very existence depends on radiation, specifically, heat and light radiation. Our dependence on plant life is thankful for ultraviolet radiation. Without visible light, you would not be able to see what is in front of you. However, in excessive amounts each of the above examples of radiation may be harmful to living organisms. Too much heat will burn you. Too much light may blind you. Strong, concentrated microwaves may be unhealthy. Nuclear radiation may also be harmful.1
In order to understand the properties of nuclear radiation and radioactivity, it is necessary to understand the basic structure of matter. An understanding of the basic structure of matter is necessary since nuclear radiation comes from matter, specifically, radioactive matter. Although an understanding of the basic physical properties of nuclear radiation and radioactivity is important to the objectives of this text, the vast minute details of atomic structure, radiation, and radioactivity are not important and are outside the scope of this text. Some of the material of this Chapter is conceptual simplistic condensation and interpretation of scientific findings. Applicability and technical accuracy of the material in this Chapter regarding the objectives are not lost due to the interpretations and condensations.
Section 2 Matter
Everything that has weight and occupies space is called matter. This book is matter. The print on this page is matter. The shoes on your feet are matter. The ground you walk on is matter. The water you drink is matter. The air you breathe is matter. Hidden in the above examples is that matter has three states of existence; solid, liquid, and gas. Regardless of the state of existence, matter has basic building blocks called elements. In order to form the myriad of substances in the universe, elements combine to form compounds. Several examples of elements and compounds are displayed in Table 1.1.
TABLE 1.1
Examples of Elements and Compounds
Examples of Elements and Compounds

An element may be described as a substance that cannot be chemically reduced into a simpler substance. For example, if you were to dissolve the element silver in nitric acid (a chemical reaction), the result would be silver nitrate—the silver would still be silver but dissolved as a chemical part of the silver nitrate. The silver could be removed from the silver nitrate by other chemical reactions.
In a less abstract sense, if you were to cut a block of silver into a thousand pieces, the pieces would still be silver. Even the saw shavings would be silver. If you kept separating the silver shavings into smaller and smaller pieces until you reached the smallest possible particle and still be silver, the particle would be an atom of silver. The atom is best described as the smallest particle an element can be divided into and still be that element.
A compound is two or more elements chemically combined. If you were to leave silver (an element) exposed to oxygen (another element) in the air long enough, the silver and oxygen would chemically combine to form the familiar black tarnish silver oxide. Silver oxide is a compound. Silver and oxygen combining happens in the same way that iron rusts when wet.2 Rust is the compound iron oxide.
If you were to separate the silver oxide, the iron oxide, or any other compound into the smallest particle the compound could be divided into and still be that compound, the particle would be a molecule. The molecule is best described as the smallest particle a compound can be divided into and still be that compound. If you were to separate the silver oxide into silver and oxygen, the result would be two separate elements, not a compound. Figure 1.1 provides a simplified drawing of atoms as separate units and combined as compounds.
FIGURE 1.1
Atoms & Molecules
Atoms & Molecules
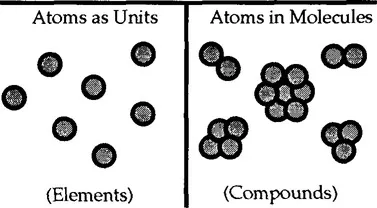
Remember:
- Elements combine to form compounds
- The smallest particle of an element is an atom
- The smallest particle of a compound is a molecule
A good understanding of the basic composition of an atom is important to understanding the nature and origin of nuclear radiation since nuclear radiation comes from radioactive atoms. A good understanding of the general structure of a molecule is necessary to understand the effects of nuclear radiation on living tissue since living tissue is comprised of a multitude of molecules.
The atom of any element can be further divided into smaller particles called subatomic particles. However, if an atom were so divided, the particles would no longer exhibit the properties of the element. Similarly, if you were to disassemble a bicycle wheel into its component parts, it would no longer be a bicycle wheel and would not function as a bicycle wheel—merely a pile of bicycle wheel parts. Now, let's take an atom apart to study the subatomic particles.
Figure 1.2 illustrates the structure of an atom as it is known today. Figure 1.2 uses helium to illustrate atomic structure. An atom is structured with a central nucleus with particles surrounding the nucleus at specific distances and in constant motion.
FIGURE 1.2
Atomic Structure
Atomic Structure

The center of the atom is called the nucleus. The nucleus contains neutrons (n) and protons (p). The nucleus is orbited by electrons (e) in particular energy shells or levels. The nucleus of any atom contains neutrons and protons except for the nucleus of normal hydrogen which contains only one proton and no neutrons.3 From Figure 1.2 remember that:
- subatomic particles are neutrons (n), protons (p), electrons (e)
- The nucleus contains neutrons and protons
- electrons surround the nucleus
Other information illustrated in Figure 1.2 includes the notation of the
- element symbol, the
- atomic weight or atomic mass number ,4 and
- the atomic number.
The element symbol is one or two letters of the name of the element. For example, the symbol for cobalt is Co. The symbol for iodine is I. The symbol for uranium is U. The symbol for cesium is Cs. The symbol for zinc is Zn. The symbol for chlorine is Cl. Some element symbols may seem odd because the symbol is taken from the latin name of the element, e.g., the latin name for sodium is naturium so the symbol for sodium is Na.
The atomic mass number (or just atomic mass) of the element is the sum of the masses of particles in the nucleus in an atom of the element, specifically, the total mass of the protons and neutrons in the nucleus. The unit for measuring the mass of any subatomic particle is the atomic mass unit (amu). The notation of atomic mass is shown in the upper left corner of the element symbol. Helium has a mass of 4 atomic mass units since it has 2 neutrons and 2 protons.5
The atomic number of an element is the number of protons in the nucleus of an atom of the element. Notation of the atomic number of an element is shown in the lower left corner of the element symbol. The atomic number for helium is 2. Other examples of element notation follow.
| Carbon (6n, 6p) | |
| Lawrencium (154n, 103p) | |
| Oxygen (8n, 8p) |
The neutron is a subatomic particle found in the nucleus. The total of neutrons in the nucleus contributes to approximately half of the mass of the atom. The mass of the neutron is approximately 1 amu. Also, the neutron has no electrical charge. The concept of electrical charge on subatomic particles will make more sense as you study the proton and electron.
The proton is the other subatomic particle found in the nucleus and also has a mass of approximately 1 amu. The total number of protons in the nucleus also contributes to almost half the mass of the atom. Where the neutron has no electrical charge, the proton has a positive electrical charge. Many things have electrical charge. Household current has electrical charge. You may build up a static electric charge when you walk across a carpet in dry humidity. Lightning is electrical charge. Just as the volt is used to express electricity, the electrical charge on the proton is expressed in electrostatic units (esu).6 One esu is equivalent to 299.8 volts. The esu charge on the proton is equivalent to 0.000000144 volt, or 0.144 millionths of one volt. It takes the charge of 2,082,000,000 protons or electrons to equal one esu.
Orbiting the nucleus of the atom are electrons. Each electron has approximately 1/1845 amu, so the electrons contribute very little to the total mass of an atom. The electrons orbiting the nucleus and their configuration around the nucleus determines the chemical properties of the parent element. How the electrons determine the chemical properties of the parent atom will be explained further as you proceed through this text. The electron has a negative electrical charge. The negative charge of the electron is exactly equal to the magnitude or strength of the positive charge on the proton but is exactly opposite in polarity. The magnitude of the charge of the electron and the proton are so exactly equal that if they were combined, their charges would completely cancel and become neutral. A proton and an electron combined as on...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Contents
- ABOUT THE AUTHOR
- ACKNOWLEDGEMENTS
- NOTE
- PREFACE
- COMMENT
- TEXT PREPARATION
- Chapter One Basic Radiological Science
- Chapter Two Exposure and Contamination
- Chapter Three Radiation Injuries
- Chapter Four Radiation Detection
- Chapter Five Recognition of Radioactive Materials
- Chapter Six Extrication and Treatment
- Chapter Seven Disrobing the Radio Actively Contaminated Patient and Radiological Survey Techniques
- Glossary
- Index