
eBook - ePub
Chemical Ecology of Insects
Applications and Associations with Plants and Microbes
This is a test
- 296 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Chemical Ecology of Insects
Applications and Associations with Plants and Microbes
Book details
Book preview
Table of contents
Citations
About This Book
Insects have evolved very unique and interesting tactics using chemical signals to survive. Chemical ecology illustrates the working of the biological network by means of chemical analyses. Recent advances in analytical technology have opened the way to a better understanding of the more complicated and abyssal interactions of insects with other organisms including plants and microbes. This book covers recent research on insects and chemical communications and presents the current status about challenges faced by chemical ecologists for the management of pests in agriculture and human health.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Chemical Ecology of Insects by Jun Tabata, Jun Tabata in PDF and/or ePUB format, as well as other popular books in Medicine & Biochemistry in Medicine. We have over one million books available in our catalogue for you to explore.
Information
PART–A
Chemical Ecology of Insects and Associated Plants and Microbes
CHAPTER
1
Plant Secondary Metabolites in Host Selection of Butterfly
Graduate School of Biosphere Science, Hiroshima University 1-4-4 Kagamiyama, Higashi-Hiroshima City, Hiroshima 739-8528, Japan E-mail: [email protected]
Abstract
Plant secondary metabolites have a role in defense against various herbivores, but they also often attract particular species and stimulate their oviposition and feeding behavior. Most butterflies are phytophagous in the larval stage and have a strong relationship with a narrow range of plants with a phylogenetic relationship and chemical similarity. Because larvae and females can respond to various plant chemicals using chemotactile (gustatory) receptors, behavioral and sensory adaptation to particular compounds are presumed to lead to the evolution of host specificity. From a limited range of butterfly species in the Pieridae, Papilionidae and Nymphalidae, a variety of plant components have been identified as semiochemicals for host selection, the majority of which are associated with female oviposition. The oviposition stimulants identified so far are (i) single compounds in relation to plant defensive chemicals or (ii) multiple components including relatively ubiquitous derivatives from primary metabolites. The finding that several closely related butterfly species use the same or corresponding compounds in different plants for host acceptance in part supports a putative evolutionary pathway in species diversification and host shift at the tribe or subfamily level.
1. Introduction
Plants biosynthesize a diverse and complex range of secondary metabolites as chemical barriers against herbivory (Rosenthal and Berenbaum 1991, Mithöfer and Boland 2012). Plant defensive chemicals are not noxious to all herbivore species, and instead are often highly and selectively toxic to particular species or otherwise have some negative effects on their acceptability and performance. Therefore, most phytophagous insects are specialized to feed on a narrow range of plant species as a result of their physiological adaptations to particular defense chemicals (Städler 1992, Bernays and Chapman 1994). From chemical interactions between angiosperms and butterflies, Ehrlich and Raven (1964) proposed a well-known hypothesis of stepwise coevolution between plant chemical defense to herbivory and herbivore counter-adaptation to plant chemicals, which is regarded as a principal driving force of species diversification from each other. Since then, plant–butterfly interactions have received the most attention as an attractive model for ecological and evolutionary studies of plant chemicals implicated in host preference and specificity of phytophagous insects.
Most butterflies are phytophagous insects in the larval stage, in which they feed on particular plant species from only one or a few genera or a single family or subfamily (Ehrlich and Raven 1964, Janz and Nylin 1998). Of a wide variety of physiological and ecological factors, non-volatile plant chemicals play a significant role to limit the host range of butterflies because these substances directly mediate and regulate the behaviors of larval feeding and female oviposition (Thompson and Pellmyr 1991, Honda 1995). Therefore, suitable host plants are chemically characterized by possessing compounds that act as behavioral stimulants and lacking those act as behavioral deterrents. An array of host plants for a particular herbivore species, in general, exhibit chemical similarity, which is often strongly associated with historical patterns of host shifts within herbivore lineages (Becerra 1997). Comparative phylogenetic studies have proposed several possible pathways of diversification and switching of butterfly hosts at the within-family or subfamily level (Janz et al. 2001, Fordyce 2010, Ferrer-Paris et al. 2013). However, only a limited number of semiochemicals in relation to host selection have been identified in a few butterfly families. The purpose of this review is to compile available knowledge on plant chemicals that serve as chemotactile (gustatory) cues for host selection by butterflies and to discuss chemical trends in their host usage.
2. Contact Chemoreception of Plant Chemicals
Typical gustatory sensilla of butterflies appear as hair- or peg-like structures consisting of a thick cuticular wall surrounding an inner lumen with the dendrites of 2–4 receptor neurons (Mitchell et al. 1999, Chapman 2003, Kvello et al. 2006). The apical part of the cuticle is perforated, allowing non-volatile compounds to enter the lumen and stimulate the receptor neurons. Each receptor neuron responds to a broad range of chemicals or only to a specific type of molecule, and conveys information concerning these particular substances to the central nervous system (CNS) separately from other kinds of information. The information from single receptor neurons receiving particular molecules serves as a stimulatory or deterrent trigger for particular behaviors, such as female oviposition and larval feeding. This is called labeled line coding in the insect taste receptor system, in which particular plant secondary metabolites serve as host-indicating signals.
In butterflies, female adults encounter more plant species than larvae because of their high mobility. Therefore, host selection (oviposition) by females is more critical for offspring than that (feeding) by larvae. Gravid females depend on a wide variety of sensory cues to recognize available host plants (Renwick and Chew 1994, Carrasco et al. 2015). In searching and orientation at long distance, olfactory and visual cues from host plants play important roles. After alighting on a plant, the forelegs make contact with the leaf and other parts of a plant in order to receive both physical and chemical cues. This behavior is called drumming, in which they respond to various plant chemicals mainly with hair-like sensilla (sensilla trichodea) on the surface of foretarsi.
Like ovipositing females, larvae also use various sensory cues to assess plant food quality. Olfaction is used for initial orientation to and discrimination of possible hosts, whereas gustation is used to determine and initiate feeding (Hanson and Dethier 1973). Freshly hatched larvae feed first on its eggshell and then on plant tissues around the egg. Because plant tissues, even in their host plants, sometimes contain toxic or growth-inhibitive components, larvae need to avoid intake of these noxious substances. They mainly use peg-like sensilla (sensilla styloconica) on the maxilla for the assessment of plant chemistry (Frazier 1992).
3 Host Selection in Pieridae
The family Pieridae is a relatively small group in butterflies, consisting of about 1100 species in four subfamilies: Pseudopontiinae, Dismorphiinae, Coliadinae, and Pierinae (Ehrlich 1958, Braby 2005). The subfamilies of the Pieridae show a distinct host specialization, in which their host plants are mainly distributed in the Fabales, Brassicales, and Santalales (Ferrer-Paris et al. 2013). Of the three plant orders, Fabales is assumed to be the ancestral host of the Pieridae, and is mainly utilized by the subfamilies Dismorphiinae and Coliadinae. Within the subfamily Pierinae, a host shift from Fabales to Brassicales, followed by further shifts from Brassicales to Santalales, had promoted diversification and adaptive radiation (Braby and Trueman 2006). The resting subfamily Pseudopontiinae, consisting of only one species, exploits the family Opiliaceae in the Santalales. The plant secondary metabolites involved in host selection have been identified to date in the genus Pieris in the Pierinae and the genera Colias and Eurema in the Coliadinae.
3.1 Pierinae and Brassicaceae
The plant family Brassicaceae is well known to produce glucosinolates, which have been studied intensively as mediators of various insect–plant interactions (Hopkins et al. 2009). When plant tissues are damaged, glucosinolates are hydrolyzed by myrosinases, and breakdown products such as isothiocyanates, nitriles, and oxazolidinethiones serve as a chemical barrier against herbivores (Winde and Wittstock 2011). The genus Pieris is a Brassicaceae-feeding specialist and closely associated with glucosinolates for their host selection. Gravid females, in the recognition of host plants, detect glucosinolates with tarsal contact chemoreceptors (Ma and Schoonhoven 1973, Du et al. 1995, Städler et al. 1995). Pieris butterflies evaluate the quality and quantity of plant glucosinolates and differ in oviposition preference and acceptance within Brassicaceae hosts (Huang and Renwick 1993, 1994). For example, Pieris rapae preferentially lays eggs on cultivated cruciferous plants such as cabbages, whereas Pieris oleracea (formerly Pieris napi oleracea) mainly exploits wild cruciferous plants such as Arabis spp. An aromatic glucosinolate, 3-indoylmethyl glucosinolate (glucobrassicin, 1) contained in cabbage, is stimulatory for the oviposition of P. rapae and Pieris brassicae (Traynier and Truscott 1991, Loon et al. 1992, Renwick et al. 1992). On the other hand, particular alkyl and thioalkyl glucosinolates, allyl glucosinolate (sinigrin, 2) and (R)-3-methylsulfinylpropyl glucosinolate (glucoiberin, 3), serve as strong oviposition stimulants for P. oleracea (Huang and Renwick 1994). In an oviposition choice between cabbage and wintercress (Barbarea vulgaris), P. rapae shows no preference, whereas P. oleracea prefers B. vulgaris. The oviposition preference of P. oleracea for B. vulgaris is attributed to a higher abundance of (2R)-hydroxy-2-phenylethyl glucosinolate (glucobarbarin, 4) (Huang et al. 1994).
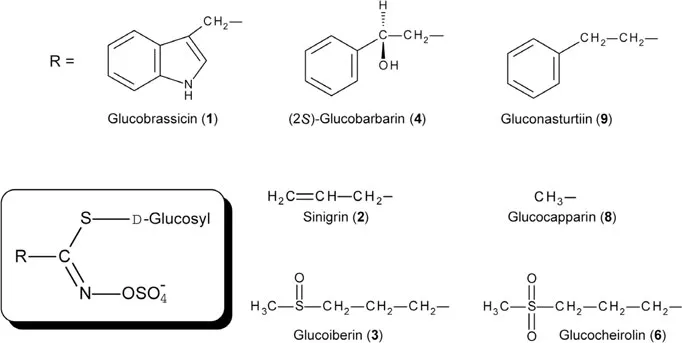
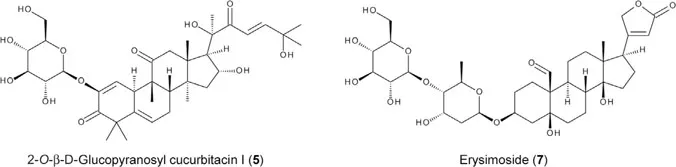
Several cruciferous plants possess oviposition deterrents for Pieris butterflies. Candytuft Iberis amara is omnipresent in grain fields in Europe and is sometimes observed to have eggs of P. oleracea. In addition to sinigrin, this plant is found to biosynthesize cucurbitacins (5), which are strong oviposition deterrents for P. rapae but only weak deterrents for P. oleracea (Huang et al. 1993a). Wormseed mustard Erysimum cheiranthoides is a preferential host for P. oleracea due to the presence of thioalkyl glucosinolates, e.g., 3-methylsulfonylpropyl glucosinolate (glucocheirolin, 6) and glucoiberin (Huang et al. 1993b). However, P. rapae never oviposits on E. cheiranthoides because its cardenolide components including erysimoside (7) serve as strong oviposition deterrents (Renwick et al. 1989, Sachedev-Gupta et al. 1990).
Pieris larvae show feeding responses to filter papers treated with cruciferous plant sap (Verschaffelt 1910). Larval feeding is stimulated by plant glucosinolates, which are received by chemoreceptors on the maxillary sensilla (Schoonhoven 1969). Particular alkyl and thioalkyl glucosinolates, e.g., methyl glucosinolate (glucocapparin, 8) and glucoiberin, elicit larval feeding by P. brassicae (David and Gardiner 1966), whereas an aromatic glucosinolate, 2-phenethylglucosinolate (gluconasturtiin, 9), stimulates feeding behavior of P. rapae (Miles et al. 2005). In several Pieris butterflies, the preference for glucosinolates is similar in both feeding and oviposition, suggesting that a common chemosensory system is involved in host selection at both larval and adult stages (Chew and Renwick 1995, Renwick 2002).
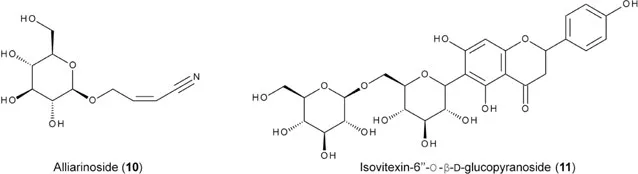
Particular cruciferous plants have feeding deterrents and sometime toxic substances for Pieris larvae. Glucosinolates, when present in plant tissues at high concentrations, are toxic to neonate Pieris larvae (Rotem et al. 2003). Several fractions of plant extract of E. cheiranthoides contain particular cardenolides, which strongly deter larval feeding of P. rapae (Dimock et al. 1991, Sachdev-Gupta et al. 1993a). Garlic mustard (Alliaria petiolate) is resistant to P. oleracea, in which a cyanoallyl glucoside (alliarinoside, 10) and an apigenin flavonoid (11) are responsible for blocking larval feeding (Haribal and Renwick 2001, Haribal et al. 2001, Renwick et al. 2001). This plant has invaded the habitat of Pieris virginiensis in North America. Because sinigrin and alliarinoside are present together at high concentrations, A. petiolate is a preferential host for P. virginiensis females but a toxic plant for its larvae (Davis and Cipollini 2014, Davis et al. 2015). Pieris larvae receive feeding deterrents with different chemical structures, particular glucosinolates and flavonoids, with the same chemoreceptor (Zhou et al. 2009).
3.2 Coliadinae and Fabaceae
Most species belonging to the subfamily Coliadinae are Fabaceae-feeders. Colias erate poliographus mainly exploits herbal fabaceous plants, especially white clover Trifolium repens. In the oviposition of C. erate poliographus on this plant, d-(+)-pinitol (12) is a principal stimulant, whereas cyanogenic glucosides (13, 14), methyl-d-glucoside, and glycerin synergistically enhanced the stimulatory effect of d-(+)-pinitol (Honda et al. 1997a, 2012). Of note, d-(+)-pinitol also stimulates the oviposition behavior of Eurema mandarina on leaves of Albizia julibrissin and Lespedeza cu...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Part A: Chemical Ecology of Insects and Associated Plants and Microbes
- Part B: Applications of Insect Chemical Ecology to Agriculture, Environment Conservation, and Public Health
- Index