
eBook - ePub
Molecular Plant Virology
Volume I: Virus Structure and Assembly and Nucleic Acid-Protein Interactions
This is a test
- 238 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Molecular Plant Virology
Volume I: Virus Structure and Assembly and Nucleic Acid-Protein Interactions
Book details
Book preview
Table of contents
Citations
About This Book
In calling this series Molecular Plant Virology, I had in mind aspects of plant virology of interest to biochemists, molecular geneticists, biophysicists, genetic engineers, or, collectively, molecular biologists. At the same time, the intention was to provide up-to-date reviews, by expert contributors, on current research topics in plant virology of interest and referential use to virologists and plant biologists. The selected topics are pitched mainly at a research level, but with sufficient introduction and cross-referencing to enable graduate students to enter this fascinating field and, hopefully, not get lost.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Molecular Plant Virology by Jeffrey W. Davis in PDF and/or ePUB format, as well as other popular books in Ciencias biológicas & Botánica. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
THE DEVELOPMENT AND APPLICATION OF ELECTRON MICROSCOPY TO THE STRUCTURE OF ISOLATED PLANT VIRUSES
TABLE OF CONTENTS
I. | Introduction | ||
II. | Development of Electron Microscope Techniques | ||
A. | Shadow-Casting Method and Replicas | ||
B. | Thin Sections | ||
C. | Evaporated Carbon Films | ||
D. | Positive and Negative Staining Methods | ||
III. | Symmetry in Virus Architecture Revealed by the Electron Microscope | ||
IV. | Terminology | ||
V. | The Extraction of Information from Electron Micrographs of Virus Particles | ||
A. | Photographic Averaging | ||
B. | Optical Diffraction Methods Applied to Electron Micrographs | ||
C. | Computer Image Reconstruction from Electron Micrographs | ||
D. | The Processing and Analysis of Images by Computer Methods for Modeling Viruses and Other Biological Structures | ||
VI. | Radiation Damage to the Specimen | ||
VII. | The Formation of the Crystalline and Paracrystalline Arrays of Plant Viruses | ||
VIII. | The Application of Electron Microscopy to the Study of Dissociated and Reassembled Products from Plant Viruses | ||
IX. | The Examination of Isolated Viral Nucleic Acids | ||
X. | Electron Microscopy of Partially Degraded Virus Particles | ||
XI. | Electron Microscope Studies of Reassembled Virus Products in Vitro | ||
A. | Isometric Viruses | ||
B. | Rod and Flexuous Viruses | ||
XII. | Low Temperature Transmission Electron Microscopy | ||
XIII. | Abbreviations Used | ||
References | |||
I. INTRODUCTION
In view of the scope of contributions to this volume on the structure, composition, and function relating to a range of plant viruses, it is an appropriate time to review the contribution electron microscopy has made to virology, with special reference to plant viruses. It is not possible to cover the voluminous literature on the electron microscopy of plant viruses, but certain landmarks were established which allowed some remarkable progress to be made up to 1984.
The published work on electron microscopes from 1932 to about 1940 was mainly concerned with the design and construction of the instrument together with improvements in magnetic and electrostatic lens systems. There were very few reports on applications to biological specimens, with only a few electron micrographs showing bacteria or viruses. It was clear that biological material was relatively transparent to the electron beam, resulting in very poor contrast causing difficulties in focusing the image. One of the earliest attempts as an aid to focusing was the deposition of colloidal gold onto the same specimen. The electron micrograph of tobacco mosaic virus (TMV) taken between 1938/39 using one of the early serially produced Seimens electron microscopes is an example of this simple technique (see Ruska,1 p. 73). Although the specimen was unstained and without any means of contrast enhancement, slender rods were faintly visible, with a size range of about 20 nm across and up to several hundred nanometers long. Among the first attempts to photograph some of the small spherical viruses (e.g., turnip yellow mosaic virus, TYMV) in the transmission electron microscope, were the experiments reported by Cosslett and Markham2 in 1948). Their electron micrographs of unstained viruses showed small areas of paracrystalline arrays of the particles when dried down onto thin plastic support films.
II. DEVELOPMENT OF ELECTRON MICROSCOPE TECHNIQUES
During the period from 1939 to 1947 the interest in the design and construction of electron microscopes increased considerably. Instruments were designed and built in Sweden, Canada, Japan, U.S., France, Switzerland, and England (see Gabor,3 Mulvey4). At the same time efforts were being made to interest the biologists in these new instruments, but the problem of contrast remained. Moreover, the methods for preparing specimens from tissues had to wait for some considerable time. Small isolated particles in the form of protozoa, bacteria, and a few viruses, on the other hand, could be studied as whole mounts by placing liquid droplets onto supports and air-dried.
A. Shadow-Casting Method and Replicas
One of the most important techniques to be developed was the deposition of heavy metal atoms by vacuum evaporation introduced by Williams and Wyckoff.5 A short piece of gold or gold/palladium alloy wire was placed on a v-shaped tungsten strip or wire, which was electrically heated in a vacuum chamber to allow the heavy metal gold atoms to coat the specimen surface at a low angle of incidence. The part of the specimen facing the source was coated leaving a “shadowed” region on the opposite side. If the approximate distance and angle of the specimen from the source was known, then the length of shadow could be calculated to give some indication of the height of the object above the surface.
In addition to determining the approximate height of specimens, the electrons scattered from the areas of heavy metal deposits resulted in considerable contrast enhancement. A typical example of a plant virus preparation prepared by shadow-casting is illustrated in Figure 1. This preparation method is still routinely used in many electron microscope laboratories. The introduction of shadow-casting coincided with better methods for the isolation and purification of several plant viruses, allowed direct approximate measurements, and determination of their shape to be made.
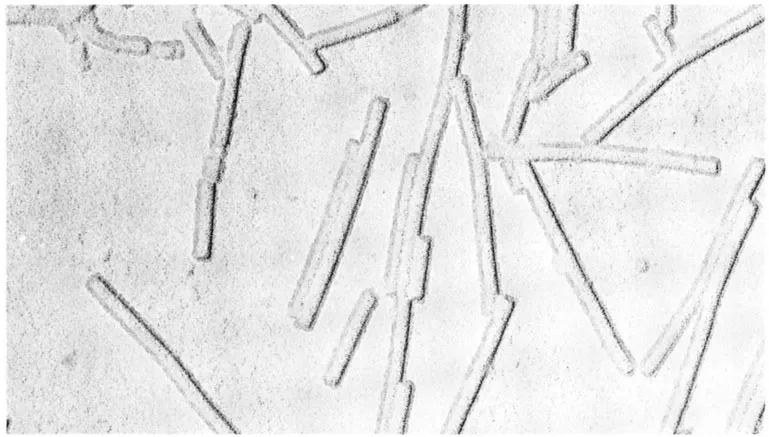
FIGURE 1. The electron micrograph shows TMV rods prepared from a liquid suspension, air dried, and shadowed. The approximate size and shape of the particles are well defined, together with some indication of their height determined from the length of shadow. No fine detail of the axial periodicity or central hole can be seen in this type of preparation. It is of interest to compare this image with the structural features of the same virus illustrated in Figure 13. (Magnification × 180,000.) (Courtesy of G. J. Hills, John Innes Institute, Norwich, U.K.)
Remarkable advances were also being made in the design and construction of electron microscopes and their lens systems. Several commercial instruments became available with potential performances approaching 1- to 2-nm resolution which were well beyond the detail visible from biological material. However, shadow-casting became a powerful method for the examination of isolated animal, plant, and bacterial virus particles, but the detail below about 3- to 4-nm resolution could not be resolved. This lack of information was attributed to possible collapse of the molecular structure during the final stages of dehydration at atmospheric pressure. Although the shape and dimensions of TMV rods were clearly seen in shadowed preparations, the axial periodicity of 2.3 nm and repeat of 6.9 nm, which was deduced from the early X-ray diffraction studies6,7,8,27 at the time, was not visible in the electron micrographs. It became clear that the TMV rod with a 2.3-nm periodicity was to provide a good test for any preparative method for electron microscopy.
Equally spectacular during the time of these early experiments on visualizing plant viruses was the introduction of two additional techniques which also used the principles of shadow-casting. First, was the procedure of Backus and Williams9 to prepare plant virus specimens by spraying droplet patterns onto a cooled surface held at about -70°C and placed in a glass apparatus which was subsequently sealed and the air pressure reduced to about 10-4 Torr. The temperature of the frozen specimen was raised to about -20°C and then continually pumped to remove water vapor. Thus a very effective and simple freeze-drying method became available for preparing purified samples of plant and other viruses. There were two advantages with this technique which enabled specimens in the form of virus particles to be viewed in various orientations with respect to the specimen plane. TMV rods for instance, were oriented parallel to the support films or seen end-on. The other advantage was that the technique provided a method for counting virus particles from liquid suspensions when mixed with polystyrene latex particles of known concentration and volume.
The second major development was the extraction shadow-replica method of Price and Wyckoff,10 and Markham et al.11 As a result of this technique, it became possible to prepare samples of small three-dimensional microcrystals of plant viruses, including TYMV and tobacco necrosis virus (TNV). It should be mentioned here that these experiments were the first attempts to relate some of the electron microscope images to data derived from X-ray diffraction studies. However, the basic problem of limited information was not solved by the freeze-drying method or shadowed replicas; the structural features were restricted to about 3- to 4-nm resolution. Moreover, apart from making direct measurements from plates or prints, there were few available procedures for allowing quantitative assessments to be made of the images recorded in the electron microscope.
B. Thin Sections
Another important advance was also introduced during the early part of 1950, when it became possible to cut thin sections from fixed and embedded bulk biological material. Although the morphology of plant viruses observed in infected plant cell systems is outside the scope of this contribution which is concerned with the structure of isolated viruses, it should be mentioned that this technique provided a major step forward in cell ultrastructure showing the location of viruses in infected cells. It was realized early in the development of the preparative techniques, coupled with the total thickness of t...
Table of contents
- Cover
- Title Page
- Copyright Page
- Table of Contents
- Chapter 1 The Development and Application of Electron Microscopy to the Structure of Isolated Plant Viruses
- Chapter 2 Structure and in Vitro Assembly of Tobacco Mosaic Virus
- Chapter 3 Structure and in Vitro Assembly of Papaya Mosaic Virus
- Chapter 4 Structure and in Vitro Assembly of Southern Bean Mosaic Virus, in Relation to That of Other Small Spherical Plant Viruses
- Chapter 5 Interaction of Alfalfa Mosaic Virus Nucleic Acid and Protein
- Index