
This is a test
- 476 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
This book comprehensively covers the chemical and physical properties and manufacturing and handling procedures of glycerine and the use of this material in cosmetic and personal care products and in other industrial areas such as testing laboratories and manufacturing and marketing sectors.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Glycerine by Eric Jungermann, Norman O.V. Sonntag, Eric Jungermann in PDF and/or ePUB format, as well as other popular books in Medicine & Dermatology. We have over one million books available in our catalogue for you to explore.
Information
1
Introduction
Jungermann Associates, Inc., Phoenix, Arizona
Glycerine was discovered more than two centuries ago by the Swedish chemist Scheele (1742–1786) when he heated a mixture of litharge (lead oxide) and olive oil. He extracted and isolated a sweet tasting liquid which he named “sweet oil” (Oelsuess). The French chemist Michel Eugene Chevreul (1786–1889), the father of the chemistry of fats and oil, established the structure of fats as triesters made up of three moles of mixed fatty acids and one mole of “sweet oil,” which he renamed “glycerine” after the Greek word for “sweet.”
Glycerine (or glycerin) is also referred to in many texts as “glycerol.” Chemically it is a tribasic alcohol and more correctly named 1,2,3-propanetriol. Its chemical structure is shown below:
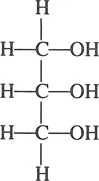
A ubiquitous substance, glycerine is found widely in nature as a component of thousands of natural substances, which have a broad range of uses in their own right; when isolated in its free form, glycerine itself has found use in a myriad of applications. As a chemical building block, it has been converted into many derivatives with additional applications. Table 1.1 describes sources and uses of glycerine and its derivatives. Table 1 also shows some of the uses and fields of application of three categories of glycerine. The listings are not intended to be all inclusive; the table is primarily designed to illustrate the broad spectrum of products and industries that use glycerine or glycerine-related materials.
Table 1.1 Sources and Uses of Glycerine and Glycerine Derivatives
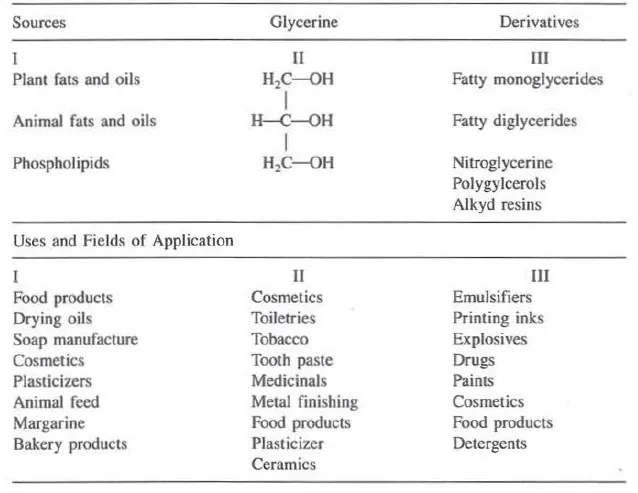
Glycerine is a versatile chemical. It is found in baby care products and in embalming fluids used by morticians, in glues that hold things together and in explosives to blow them apart; in throat lozenges and in suppositories. A colorless, viscous liquid, and stable under most conditions, glycerine is nontoxic, easily digested, and is environmentally safe. It has a pleasant taste and odor, which makes it an ideal ingredient in food and cosmetic applications.
Production of glycerine has been relatively stable in recent years, as shown in Table 1.2.
Table 1.2 U.S. Production of Glycerine
Year |
lbs. (MM) |
1987 |
307.2 |
1988 |
296.8 |
1989 |
293.7 |
Source: From Ref. 1.
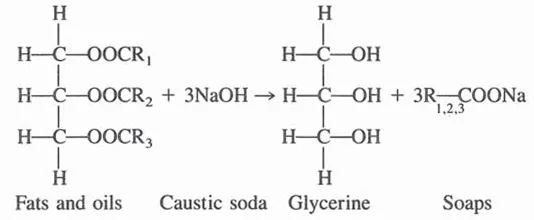
The largest quantity of naturally occurring glycerine is obtained from (a) the manufacture of soap and (b) the production of fatty acids by fat splitting (hydrolysis).
Soap is manufactured by reacting fats and oils with caustic soda [2]
Production of fatty acids by fat splitting (hydrolysis) is shown below:
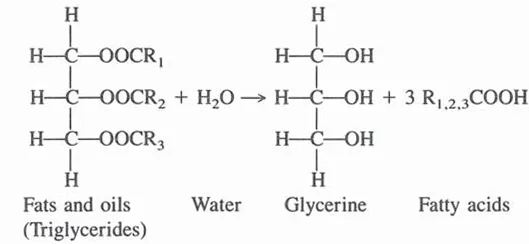
Some fatty acids obtained by fat splitting are neutralized to make soaps; they also have many applications per se, or are used in the manufacture of derivatives. Another process that results in the formation of glycerine from fats and oils occurs in the manufacture of methyl esters of fatty acids. In this process fats are interesterified with methyl alcohol:
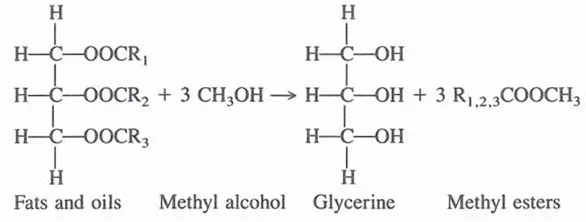
Methyl esters are useful in the manufacture of many fatty derivatives, such as fatty alcohols and amides.
The first part of this volume provides a broad background on the properties of glycerine, methods of manufacture, and some of the historical and economic aspects of this important chemical. Chapter 3 discusses the various processes for producing glycerine from natural fats and oils. Methods of separation from the resultant fatty residues and subsequent purification techniques are reviewed. Chapter 4 is concerned with a review of various industrial methods for manufacturing glycerine from nonfat-based starting materials. Processes utilizing petrochemicals constitute the most important examples, although the chapter will also cover natural carbohydrates as raw materials. The production of synthetic glycerine from petrochemicals had an important impact on that industry in the preoil crisis years of the 1960s. Dow, FMC, Olin, and Shell operated plants in the United States engaged in producing synthetic glycerine. Shifting economic climates have shut down most of these plants. In 1990, only Dow still produces synthetic glycerine in the United States.
Chapters 5, 6, and 7 deal with the chemical and physical properties of glycerine. The chemical reactions of glycerine as used in the production of industrially important derivatives are relatively simple and are discussed in some detail in Chapter 5, as well as in Chapter 12, which is specifically concerned with monoglycerides. Many reactions of historical and scientific interest are also reviewed in Chapter 5. Chapter 6 discusses the physical properties of glycerine. The multiplicity of uses of glycerine are not due to a single property, but rather to a unique combination of properties, such as broad compatibility, blandness, nontoxicity, stability, and hydroscopicity, These are the key physical properties that make glycerine an almost essential ingredient in cosmetic formulations.
The various methods for analyzing glycerine are covered in Chapter 7. These include the traditional “wet” methods, as well as modern instrumental techniques. The analyses used for the detection of various impurities that can be found in glycerine are also discussed. A review on handling, safety, and environmental factors of glycerine are described in Chapter 8. A chapter on economic implications concludes the first part of the volume.
The second half of the text deals with the broad role and the many applications of glycerine in the cosmetics and toiletries industry. Chapters 10 and 11 discuss the functions glycerine fulfills in various formulations, and evaluation techniques used to demonstrate efficacy. Chapters 13, 14, and 15 cover specific product areas, such as skin, hair, and oral care products, and soaps. The role of glycerine in the formulations of these products is discussed in detail. Chapter 16 considers some alternatives to glycerine use in cosmetic formulations. The volume concludes with a broad-based review of some of the other fields and areas where glycerine is widely used.
REFERENCES
1. Bureau of the Census, U.S. Department of Commerce, Current Industrial Report Series M20K, Fats and Oils.
2. Jungermann, E., Soap, in Bailey’s Industrial Oil and Fat Products, 4th ed. ( D. Swern, ed.), John Wiley & Sons, New York, 1979, Chap. 8.
2
Glycerin(e) and Its History
Henkel Corporation, Gulph Mills, Pennsylvania
As early as 600 A.D., the Phoenicians were reported to have an “alchemist knowledge” of soap making, which in later centuries found its way north via Marsilia into Gallic and Germanic customs [1]. No references are known, however, to a “by-product of soap making” named “glycerine or glycerol,” whose existence twentieth-century man has taken for granted. From pre-Renaissance Italy, references to soap making are found in Central European and German regions in later centuries. Under Charles I (14th century), the English crown monopolized the commercial trade with soap and even extracted an excise tax from people with a preference for cleanliness.
Inquisitive minds on the Continent and in England paved the way from alchemy to chemistry. A historical example is C. G. Geoffrey [2] (1741), whose intensive studies of the nature of fatty substances unlatched the door for the discovery of glycerine.
Less than 40 years later, Karl Wilhelm Scheele (1742–1786), a scientifically curious young pharmacist in Sweden, achieved the historic distinction of discovering that natural oils and fats contain what we refer to as “glycerine,” a sligh...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- About the Series Eric Jungermann
- Foreword Theodore E. Brenner
- Preface
- Contributors
- 1 Introduction
- 2 Glycerine and Its History
- 3 Manufacture of Glycerine from Natural Fats and Oils
- 4 Manufacture of Glycerine from Petrochemical and Carbohydrate Raw Materials
- 5 Chemical Reactions of Glycerine
- 6 Physical Properties of Glycerine
- 7 Glycerine Analysis
- 8 Handling, Safety, and Environmental Aspects
- 9 Economics
- 10 Functions of Glycerine in Cosmetics
- 11 Methods for Evaluating the Efficacy of Cosmetics Containing Glycerine
- 12 Chemistry and Biology of Monoglycerides in Cosmetic Formulations
- 13 Glycerine in Creams, Lotions, and Hair Care Products
- 14 Glycerine in Oral Care Products
- 15 Glycerine in Bar Soaps
- 16 Alternatives to Glycerine in Cosmetics
- 17 Other Applications of Glycerine
- Index