
- 400 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Our world is widely contaminated with damaging chemicals, and companies create thousands of new, potentially dangerous chemicals each year. Due to the difficulty and expense of obtaining accurate measurements and the unreliability of reported values, we know surprisingly little about the properties of these contaminants. Determining the properties of chemicals is critical to judging their impact on environmental quality and in making decisions about emission rates, clean-up, and other important public health issues.
Chemical Property Estimation describes modern methods of estimating chemical properties, methods which cost much less than traditional laboratory techniques and are sufficiently accurate for most environmental applications. Estimation methods are used to screen chemicals for testing, design monitoring and analysis methods, design clean-up procedures, and verify experimental measurements. The book discusses key methods for estimating chemical properties and considers their relative strengths and weaknesses. Several chapters are devoted to the partitioning of chemicals between air, water, soil, and biota; and properties such as solubility, vapor pressure, and chemical transport.
Each chapter begins with a review of relevant theory and background information explaining the applications and limitations of each method. Sample calculations and practical advice on how and when to use each method are included as well. Each method is evaluated for accuracy and reliability. Computer software, databases, and internet resources are evaluated, as well as other supplementary material, such as fundamental constants, units of measure, and more.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
CHAPTER 1
Estimating the Properties of Chemicals: The Foundation of Environmental Research
1.1 INTRODUCTION
We live in a world that is widely contaminated with high levels of chemicals that are persistent and potentially damaging to human health and welfare. Already, we spend a significant amount of time and money attempting to remedy and control pollution. And every year, the chemical industry invents thousands of new chemical products. Many are potentially dangerous; some will find their way into the environment in appreciable quantities; some will be hazardous to human health and welfare.
If we are to predict the behavior of these chemicals and control our exposure to them, we must know their physical and chemical properties. But we know all too little about the properties of the environmental contaminants we already deal with, and we know less about the new and novel chemical products introduced each year. The properties of interest to environmental specialists have not been measured for most compounds released into the environment.
Why is it that we know so little about the chemical properties of existing environmental contaminants? There are many reasons. It may be impossible to measure a chemical’s properties satisfactorily, for one thing. Perhaps an authentic sample of the compound isn’t available. Perhaps the chemical decomposes during the process of measuring its properties, a problem that arises in melting-point and boiling-point measurement, for example. Perhaps there simply isn’t a reliable way to measure the chemical property; we have only recently developed methods of measuring the very low vapor pressures and aqueous solubilities that some chemicals exhibit under ambient conditions.
Cost is another factor. Laboratory measurements take time and money; it’s impossible for environmental specialists to keep up as thousands of new chemicals are added each year to the millions already in existence. Approximately 30,000 or more commercially successful chemicals have already been released into the environment in significant amounts, and hundreds more are added to this list each year. Yet a single measurement of vapor pressure or solubility can cost thousands of dollars and take days to perform. Measuring a property such as a chemical’s Henry’s law constant, soil sorption coefficient, or bioconcentration factor can cost considerably more. The amount of reliable data available for any one chemical is likely to be limited. And since environmental conditions differ from one location to the next, and from time to time, the scanty information available will probably not be relevant to the problem at hand. For instance, the environmental specialist who wants to assess exposure to a certain chemical may need to know its vapor pressure in a mixture at 298 K but could discover that the only information available relates to the saturation vapor pressure of the pure compound at 273 K. More likely, only the melting point or normal boiling point of the chemical will be available.
If the environmental specialist doesn’t know the properties of a chemical, he or she can’t predict how it will be distributed or what its fate will be in the environment. Determining chemical properties is a key step in determining how pollutants will affect the quality of the environment. It is also a prerequisite for developing rational policies about emission rates, cleanups, and other decisions that affect public health and well-being.
Compared to most measurement methods, the computational methods of estimating chemical properties that are described in this book cost very little. When they are properly applied, they give results that are sufficiently accurate to be useful in most environmental applications. This is why estimation methods are so useful in studying the distribution, fate, and impact of environmental contaminants.
In the following sections of this chapter, we will examine the scope of chemical property estimation methods today. The chapter describes important physicochemical properties that control chemical behavior in environmental systems, as well as exploring the kinds of mathematical relationships used in chemical property estimation. We’ll discuss some details concerning units of measure, then conclude with an overview of the organization of this book.
1.2 WHY WE ESTIMATE CHEMICAL PROPERTIES
Who Uses Chemical Property Estimates?
Research scientists and engineers use chemical property estimates to get a rough idea of the behavior of a chemical. Researchers ordinarily require higher accuracy than that offered by the usual estimation method, but a rough estimate may be adequate to select chemicals for a study, to design an instrument or a measurement technique, or to investigate a suspect experimental measurement.
Regulatory personnel and environmental specialists use the information to screen chemicals for laboratory testing and to set regulatory priorities. Estimates of chemical properties are used as input to screening and management models that predict the distribution and fate of chemicals in the environment. The models are used as aids in designing environmental cleanups and in establishing permissible emission limits.
In general, experts use various stochastic (statistical) and deterministic (non-statistical) thermodynamic models to describe the way contaminants are distributed in the environment. Similarly, experts use various stochastic and deterministic kinetic models to describe the rate of transport of contaminants in the environment. These models require input of the values of various partition coefficients, diffusion coefficients, and other chemical properties.
A major problem facing environmental specialists is the difficulty of measuring key chemical properties of important classes of chemicals and the resulting unreliability of measured values reported in the literature. The unreliability of published values of aqueous solubilities and octanol-water partition coefficients of hydrophobic chemicals, for example, is frequently mentioned as a problem of special concern. As chemical property estimation methods grow increasingly sophisticated and accurate, they grow increasingly useful for identifying questionable values of chemical properties that have been published.
1.3 PREDICTING ENVIRONMENTAL PARTITIONING AND TRANSPORT
Understanding the need to estimate certain chemical properties begins with understanding, first, the way the environment itself is modeled and second, the assumptions on which our models of chemical partitioning and transport are based.
For convenience, we imagine the environment as divided into four major compartments: soil, water, air, and biota. Each compartment contains several phases: solid, liquid, and vapor. Chemicals are spontaneously transported between and within compartments from regions of high chemical potential* to regions of low chemical potential. The chemical transport processes are driven by departures from thermal, mechanical, and material equilibrium. The scheme is illustrated in Figure 1.1.
While net transport of chemicals between phases is the result of departures from equilibrium, the problem of modeling chemical partitioning is much simpler if we assume that the system is at equilibrium. The partitioning of chemicals among phases can then be modeled using estimates of gas–liquid, liquid–solid, and gas–solid partition coefficients.
Chemicals are spontaneously transported within phases along concentration gradients from areas of high concentration to areas of low concentration. Transport occurs by the processes of advection, dispersion, and molecular diffusion.** The rate of transport may be calculated with the help of estimated molecular diffusivities. Estimates of properties such as density and viscosity may also be required in order to form a detailed picture of chemical transport in the environment.
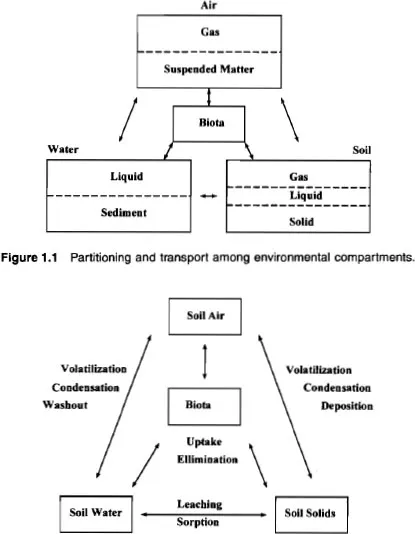
Figure 1.1 Partitioning and transport among environmental compartments.
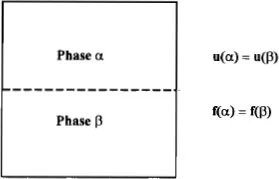
Figure 1.2 Partitioning and transport in the soil column.
Consider the partitioning and transport of chemicals deposited in a soil column as shown in Figure 1.2. The chemicals are found sorbed on soil particles and biota, dissolved in soil solution, and vaporized within soil pore spaces as well as within dislocations, worm holes, and so on. The soil sorption coefficient, the concentration ratio of chemical sorbed on soil particles to chemical dissolved in soil water at equilibrium, KSW, is often estimated with the organic carbon partition coefficient, KOC, or the organic matter partition coefficient, KOM. The partitioning of chemical species between soil water and soil air is estimated with the air–water partition coefficient (the dimensionless Henry’s law constant), KAW. Estimates of bioconcentration factors, BCF, bioaccumulation transfer factors, TF, and plant uptake factors, Bv, will also be useful in determining partitioning. If the chemical is found in an organic phase in the soil column, the chemical’s vapor pressure and aqueous solubility must be considered, also. (A useful review of chemical partitioning and transport in soils is given by Lyman et al., 1992.)
Table 1.1 Chemical Properties That Control Chemical Partitioning and Fate
Environmental Partitioning |
Boiling point Melting point Gas and liquid density Surface tension Vapor pressure Aqueous solubility Air-water partition coefficient Sorption coefficient for soil and sediment Bioconcentration factor |
Environmental Transport and Fate |
Diffusivity in air and water Phase transfer coefficient for air–water Phase transfer coefficient for air–soil |
While diffusion of sorbed species in soils is usually negligible, diffusion of chemicals in soil water and in soil air is fast enough to be important. In addition, dissolved chemicals are transported along with the soil water by wicking and percolation in the unsaturated zone and by advection and dispersion in the saturated zone. Chemicals are transported in soil air by barometric pumping resulting from sporadic changes in atmospheric pressure and by displacement with soil water. The estimated diffusivities of chemicals in air and water, along with information about the medium such as tortuosity and hydraulic conductivity, are used to evaluate chemical movement in the soil column due to the various transport processes.
Table 1.1 summarizes some important chemical properties that control the partitioning and transport of chemicals in the environment. Measured values are often available for properties such as melting point, boiling point, aqueous solubility, and octanol-water partition coefficient, although the reported values are not always reliable. Measured values of vapor pressure, air-water partition coefficient, soil sorption coefficient, bioconcentration factor, and diffusion coefficients are rarely available (Altschuh and Bruggemann, 1993). Many of these are commonly estimated from the magnitude of the chemical’s octanol-water partition coefficient, KOW. Methods of estimating the magnitude of the properties of chemicals and some key properties of environmental media are fundamental to modeling the behavior of chemicals in the environment and are presented in this book.
1.4 ORGANIZATION OF THE BOOK
The book opens with a discussion of the types of methods used to estimate chemical properties: quantitative structure-property relationships and quantitative property-property relationships. We review some of the fundamentals in order to alert you to the strengths and weaknesses of the various types of models. Several chapters follow that deal with properties controlling the partitioning of chemicals between air, water, soil, and biota. The book ends with several chapters dealing with properties that control chemical transport in the absence of equilibrium.
Each chapter begins with a review of background information and theory explaining the range of application and limitations of the methods to be described. Chemical property estimation methods are presented along with sample calculations and practical advice on how and when the methods are best used. The methods range from simple, back-of-the-envelope calculations to complex procedures requiring the aid of a computer. Most of those described apply to the wide range of chemicals that are of interest to environmental specialists. All of the...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- 1. Estimating the Properties of Chemicals: The Foundation of Environmental Research
- 2. Concepts and Theory of Chemical Property Estimation
- 3. Boiling Point and Melting Point
- 4. Density and Molar Volume
- 5. Surface Tension and Parachor
- 6. Vapor Pressure
- 7. Aqueous Solubility and Activity Coefficient
- 8. Air-Water Partition Coefficient
- 9. Octanol-Water Partition Coefficient
- 10. Soil and Sediment Sorption Coefficient
- 11. Bioconcentration Factor and Related Parameters
- 12. Diffusivity
- 13. Volatilization from Soils
- 14. Volatilization from Water
- Appendices
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Chemical Property Estimation by Edward Baum in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.