![]()
Part I
Biosynthesis, Compartmentation, and Transport of Glutathione
![]()
1 Biosynthesis of Glutathione and Its Regulation
Henry J. Forman and Hongqiao Zhang
University of California, Merced
Terrance J. Kavanagh
University of Washington
CONTENTS
1.1 Introduction
1.2 Structure of GSH
1.3 GSH Synthesis
1.3.1 The γ-glutamyl Pathway
1.3.2 Availability of Cysteine and Cystine
1.3.2.1 Cystine Uptake
1.3.2.2 N-Acetylcysteine
1.3.2.3 Methionine and the Transsulfuration Pathway
1.3.3 Regulation of Enzymatic Activity and Expression
1.3.3.1 GCL Kinetics
1.3.3.2 GCL Expression
1.3.3.3 GS Expression
1.3.4 Dysregulation of GSH Synthesis
1.3.4.1 Diseases Associated with Changes in GSH Synthesis
1.3.5 Rare Inborn Errors of Metabolism Where There Is Absence of Protein or Where Mutation Causes Substantial Reduction in Enzyme Activity
1.4 Decreased GSH Content with Aging
1.5 Summary
References
1.1 INTRODUCTION
We are honored to participate in this volume on glutathione (GSH), a subject to which the editor, Leopold Flohé, has made many outstanding contributions (Ursini and Maiorino, 2010).
This chapter will review the biosynthesis of GSH and its regulation in mammals. We refer readers to reviews on GSH synthesis in plants (May et al., 1998; Noctor et al., 1998) and yeast (Wu and Moye-Rowley, 1994) for information on organisms in those taxa. Although some bacteria synthesize GSH, many use other thiols in its place (Fahey, 2013). GSH is used in antioxidant defense, cell cycle regulation, and other important roles in cells, making it important to maintain its concentrations. Although other chapters focus on the uses of GSH, we will discuss how GSH synthesis is maintained through feedback and feedforward regulation at several levels of control.
1.2 STRUCTURE OF GSH
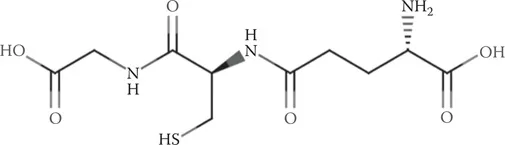
GSH is a tripeptide composed of an N-terminal glutamic acid, a central cysteine, and a C-terminal glycine (Figure 1.1). There are two interesting points about the GSH structure that contribute to its uniqueness. One is that the glutamate is attached to cysteine in an amide bond through the γ-carboxyl rather than the α-carboxyl moiety. The second unique aspect of GSH is that it contains a large percentage of the cysteine in cells, as the concentration of GSH ranges from 1 to 10 mM depending on the cell type (Meister, 1988). Indeed, when cysteine is limiting, which is the usual situation, it is preferentially used for GSH synthesis; only protein synthesis has a higher priority than GSH synthesis (Stipanuk et al., 1992). Glycine, the third amino acid in GSH, is linked to cysteine in a normal peptide amide bond. Thus, GSH or γ-L-Glu-L-Cys-Gly is an unusual peptide. But you likely already knew that if you are reading this book.
FIGURE 1.1 The structure of GSH.
1.3 GSH SYNTHESIS
The concentration of GSH is maintained by multiple mechanisms. This chapter is focused on de novo synthesis of GSH; however, perhaps the most important mechanism maintaining GSH is the reduction of glutathione disulfide (GSSG) by glutathione reductase (GR) using NADPH. GSSG is produced during the reduction of hydroperoxides by glutathione peroxidases. Although the pathway for GSH synthesis and the supply of the amino acids were largely worked out decades ago, today we are still faced with the challenge of how to most effectively elevate GSH when that would appear to help resist oxidative or xenobiotic stresses. Thus, this chapter combines the old with the new aspects of GSH synthesis, which are needed to understand its regulation.
1.3.1 THE γ-GLUTAMYL PATHWAY
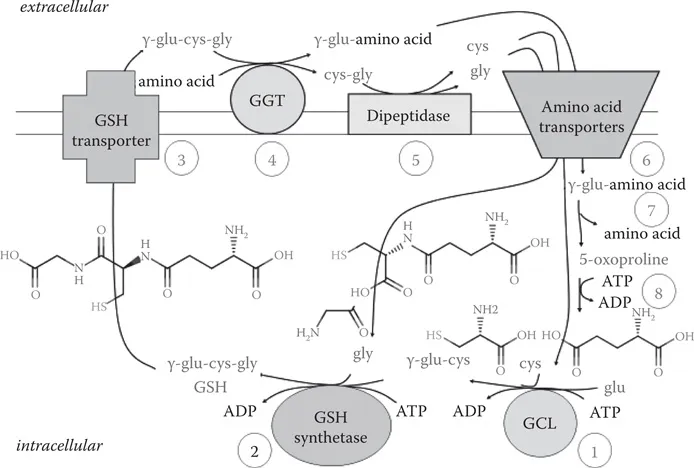
The main synthetic pathway for GSH is called the γ-glutamyl cycle, which is illustrated in Figure 1.2. In step 1, L-glutamic acid is joined to L-cysteine in an ATP-dependent reaction catalyzed by glutamate cysteine ligase (GCL). This enzyme is composed of catalytic and modifier subunits that will be described in detail next. GCL enzymatic activity is regulated by the concentrations of substrates, the feedback inhibition by GSH, and the association of the two subunits, and at their transcription and translation levels. In step 2, glycine is rapidly added to γ-Glu-Cys by forming an amide bond with the cysteine carboxyl group in a second ATP-dependent reaction catalyzed by glutathione synthase (GS). GCL is usually considered as the rate-limiting step in de novo GSH synthesis, although some evidence suggests that increasing GS can also increase GSH synthesis (Lu, 2009). These two steps would be sufficient for de novo GSH synthesis, but as Meister and coworkers reported, GSH synthesis is part of a cycle in which the glutamic acid is used to import other amino acids from the external fluid (Orlowski and Meister, 1970). Thus, in step 3, GSH is exported from cells. As the extracellular GSH concentration is in the micromolar range, the steep gradient from inside to outside drives the export of GSH. Once outside, the cysteinyl-glycine part of GSH is exchanged in step 4 for another amino acid in a reaction catalyzed by the exo-enzyme γ-glutamyl transferase (GGT; also known as γ-glutamyl transpeptidase). The cysteine and glycine from cysteinyl-glycine can be recovered through step 5 in which a dipeptidase cleaves the amide bond, followed by the uptake of the amino acids through specific transporters in step 6. The γ-glutamyl-amino acid formed in step 4 can also be transported by a transporter that is specific for γ-glutamyl-amino acids. Once inside, the γ-Glu-amino acid is converted by γ-glutamyl cyclotransferase in step 7 into the amino acid and oxoproline. Oxoproline is hydrolyzed to L-Glu in an ATP-dependent reaction catalyzed by oxoprolinase in step 8. Thus, all three amino acids are cycled preferentially providing for the restoration of GSH lost from the cell but also for the import of other amino acids. Although all the steps in the cycle are interesting in that deficiencies cause disease, we will largely focus on how GCL and GS are regulated.
FIGURE 1.2 The γ-glutamyl pathway.
1.3.2 AVAILABILITY OF CYSTEINE AND CYSTINE
Normal nutrition supplies glutamic acid and glycine in far more abundance than is needed for GSH synthesis. The availability of cysteine, however, normally limits GSH synthesis (Meister, 1981). In fact, as the KM for cysteine for GCL is near the normal physiological concentration (Richman and Meister, 1975), providing more cysteine generally increases GSH concentration. As described previously, cysteine can be recovered from the GSH that leaves cells. In the blood, cystine, the disulfide form of cysteine, is the predominant form of these two amino acids. Fortunately, the preferred amino acid acceptor of the γ-glutamyl group used by GGT is cystine (Anderson and Meister, 1983). Therefore, GGT uses GSH and cystine to produce γ-glutamyl-cystine and Cys-Gly. The γ-glutamyl-cystine is then taken up by the γ-glutamyl amino acid transporter. Once inside the cell, γ-glutamyl-cystine can be reduced to form γ-Glu-Cys by transhydrogenation with GSH to form GSSG, cysteine, and γ-glutamyl-cysteine (Anderson and Meister, 1983). GSSG is rapidly reduced in cells by GR using NADPH. The formation of γ-glutamyl-cysteine from γ-glutamyl-cystine bypasses GCL, the usually rate-limiting enzyme.
1.3.2.1 Cystine Uptake
Cystine can also be transported through the x(c)- transport system in exchange for glutamate and then reduced to cysteine and used by GCL (Deneke and Fanburg, 1989). Indeed, increasing evidence suggests that the x(c)- transport system is critical for maintenance of GSH in preventing ferroptosis in tumors (Jiang et al., 2015). Thus, cysteine can be recovered by transport of cysteine, cystine, γ-glutamyl-cysteine, or γ-glutamyl-cystine, which varies in importance among cells. Although cysteine can be synthesized from other sulfur-containing compounds described here, much of it is obtained from the diet. This classifies it as a semi-essential amino acid. The cysteine is oxidized to cystine during food preparation so that the majority of the cystine is taken up by the x(c)- transport system.
1.3.2.2 N-Acetylcysteine
N-Acetylcysteine (NAC) is an amide formed from acetic acid and cysteine that is readily taken up by cells. Inside the cells, it is rapidly hydrolyzed to form its parent compounds, providing a source of cysteine but also potentially acidifying the cytosol through the sudden increase in acetic acid. A recent study (McCarty and DiNicolantonio, 2015) suggested that cysteine from the diet may not be sufficient in the elderly but can be remedied by supplying NAC and inducers of GCL transcription (see later). The abrogation of damage by NAC is used in many studies to imply the involvement of oxidative stress. Indeed, as it is a poor scavenger of reactive species, its effectiveness more likely involves the elevation of GSH, although, as stated previously, it acidifies the cytosol.
1.3.2.3 Methionine and the Transsulfuration Pathway
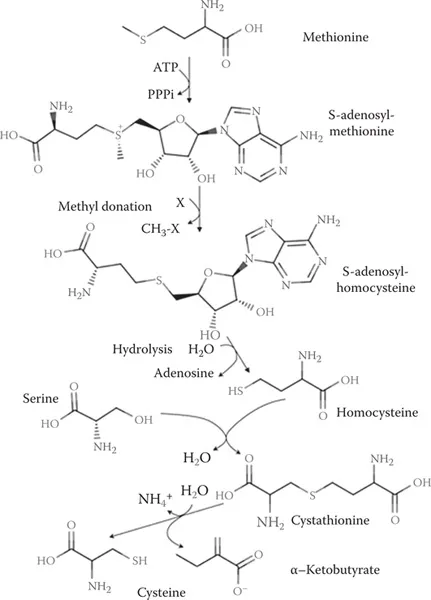
In some tissues, much of the cysteine is provided by using the essential amino acid methionine using the transsulfuration pathway (Banerjee and Zou, 2005). An excellent review by Stipanuk (2004) of the uptake of cysteine and methionine, and the transsulfuration pathway describes these important contributions in much greater detail than we can provide here. Briefly, methionine, ATP, and serine are used to produce cysteine in the scheme shown in Figure 1.3. The importance of this pathway in some tissues is apparent from the demonstration that deficiency of the final enzyme in the pathway, cystathionine γ-lyase, results in neurodegeneration in Huntington’s disease (Paul et al., 2014).
FIGURE 1.3 The transsulfuration pathway.
1.3.3 REGULATION OF ENZYMATIC ACTIVITY AND EXPRESSION
1.3.3.1 GCL Kinetics
Alton Meister’s group did most of the pioneering work on understanding the regulation of GCL activity at the enzyme kinetics level. Here, we will describe the major points from their studies (Huang et al., 1993a,b,c; Richman and Meister, 1975; Anderson and Meister, 1983). GCL activity is regulated by the availability of its substrates. But it is also regulated by the interaction between its catalytic (GCLC) and modifier (GCLM) subunits.
Let us first consider the KM’s for the substrates of the GCLC/GCLM dimer, also called the holoenzyme. The KM for cysteine for GCL activity is 0.35 mM, which is within the intracellular concentration range for this amino acid (Richman and Meister, 1975). The KM for glutamate for GCL activity is also within the intracellular concentration range for glutamate (Huang et al., 1993a,c; Chen et al., 2005). Similarly, the KM for ATP (0.87 mM) (Chen et al., 2005) is within its normal physiological range. Thus, GCL activity is regulated by the intracellular concentration of all three substrates. Nonetheless, as cysteine is the one that is most limited in terms of its availability from either the diet or its synthesis, it is considered the limiting factor, particularly during oxidative stress when GSH synthesis is generally increased.
GCL activity is feedback inhibited by GSH. But this inhibition is competitive with glutamate rather than allosteric (Richman and Meister, 1975). The Ki for GSH is 2.3 mM, which is also in the range of its intracellular steady-state concentration in most cells. Thus, it is expected that GCL operates in cells at less than the maximum rate, as feedback inhibition by GSH would be significant. The combination of substrate availability and feedback inhibition suggests that GSH synthesis is well regulated at the enzyme kinetics level.
When we consider the role of the interaction of the two subunits, regulation at the enzymatic activity level becomes more complex. Using recombinant proteins, it was shown that GCLC has GCL catalytic activity in the absence of GCLM, which itself has no catalytic activity (Huang et al., 1993a). Nonetheless, the effect of GCLM is profound, as GCLC alone has an approximately ninefold higher KM for glutamate (Huang et al., 1993a) allowing the holoenzyme to be sensitive to glutamate concentrations in the physiological range. The same study also demonstrated that GCLM significantly lowers the Ki for GSH. Later studies by Chen et al. (2005) showed that GCLM decreased the KM for ATP by approximately sixfold and confirmed the reported effects on the KM for glutamate and the Ki for GSH. Furthermore, GCLM increased the kcat of GCLC by 4.4-fold (Chen et al., 2005). During apoptosis, GCLC can be cleaved to a shorter form by caspase-3, which may cause a decline in GCL activity due to altered interaction with GCLM (Franklin et al., 2003).
Although the holoenzyme is a 1:1 heterodimer, we found that the binding of GCLM and GCLC is not 100% efficient and that the ratio of the two subunits varies among tissues (Krzywanski et al., 2004). The interaction of GCLM with GCLC due to changes in their expression has physiological implications (Chen et al., 2005; Lee et al., 2006) that are described in more detail in a later section...