![]()
1
Cardiovascular System:
Anatomy and Physiology
C. A. Gibbons Kroeker
Contents
1.1 The Heart
1.2 The Heart Valves
1.3 Electrical Activity of the Heart
1.4 The Cardiac Cycle
1.5 Blood Flow
1.6 Blood Vessel Properties
1.7 The Arterial System
1.8 The Microcirculation
1.9 The Venous System
1.10 The Blood
Acknowledgment
The cardiovascular system consists of three components: the heart, the blood, and the blood vessels. The heart behaves as a hydraulic pump, creating the driving force behind the pulsatile blood flow through the arterial system. The magnitude and behavior of this blood flow is dependent on the mechanical properties of the heart muscle and the blood vessel wall, as well as the fluid properties of the blood.
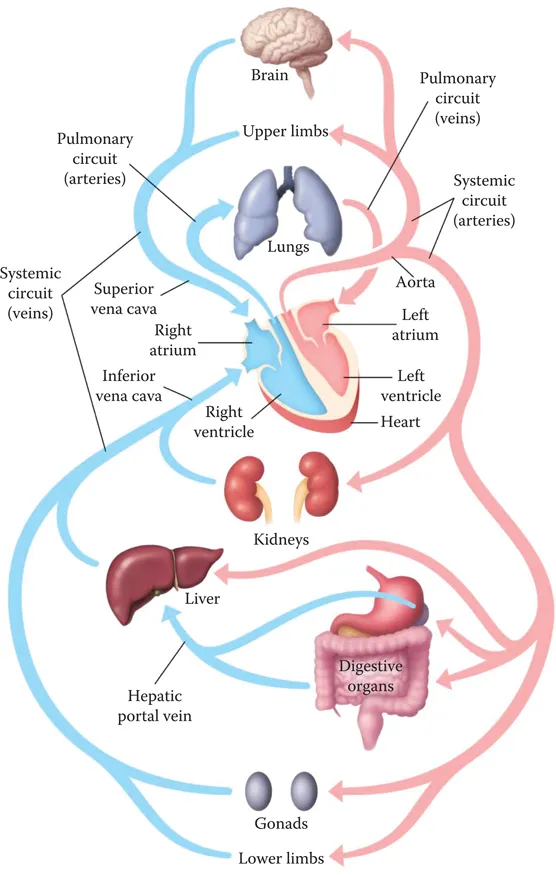
The human heart is roughly the size of a human fist, with an average mass of 275 g. It begins to beat within a few weeks of gestation, contracting approximately once per second through a lifetime (about 3 billion heart beats). The heart is a dual-pump system (Figure 1.1). The right side of the heart collects deoxygenated blood from the venous system to be sent to the pulmonary arteries and the lungs (the pulmonary circulation), while the left side collects oxygenated blood from the pulmonary veins to be sent out via the aorta to the body tissues (the systemic circulation). The pulmonary circulation has a lower pressure than the systemic circulation. It is a low-resistance system that moves 100% of the blood through the lungs, exchanging carbon dioxide for oxygen. In contrast, the systemic circulatory system acts as a parallel circuit, and the distribution of blood to each of the body organs varies, depending on the relative resistance of the arterioles and capillaries preceding the organ system. At rest, a large proportion of the total blood flow is directed to the digestive tract and the kidneys. With exercise, the arterioles to the muscle beds vasodilate, decreasing the resistance and redirecting more blood flow to this organ system, while decreasing the relative flow to the digestive tract and kidney. Although blood flow to many body organs may vary, blood flow to the brain remains constant.
Figure 1.1 The circulatory system. While the pulmonary circuit receives 100% of the blood flow, the systemic system is arranged as a parallel circuit. Relative blood flow to each organ system will depend on the vascular tone of the vessel feeding the organ.
1.1 The Heart
The heart is enclosed within the pericardium, a thin but tough connective tissue sheath. It provides protection and anchoring of the heart within the chest cavity, as well as acts as a constraint that prevents the overfilling of the heart chambers. The fluid within the pericardial sac gives the heart some lubrication as it contracts and moves within the space. The heart muscle is supplied with blood and oxygen by the coronary circulation (the right, the left anterior descending, and the left circumflex coronary arteries). During systole (or contraction), the vessels are compressed by the muscle, so most of the coronary flow (roughly 70%) must occur during diastole (or relaxation). If nutrient supply is insufficient (e.g., by an occluded coronary artery in ischemia), damage to the heart muscle can occur.
The heart muscle is made up of cardiac muscle cells connected by intercalated discs. These discs consist of two cell junctions—a desmosome, which can withstand mechanical stress, and gap junctions, which allow for the movement of nerve impulses between cells. The muscle cells are arranged in a spiral pattern that results in ventricular torsion as the muscle contracts. It is believed that this “twisting” action aids in the ejection of blood, much like the effect seen when wringing out a wet cloth. In the isovolumic relaxation period, the muscle relaxes and the ventricular volume expands. This resulting drop in pressure may also aid in ventricular filling (known as the “diastolic suction” effect).
The heart is made up of four chambers—the left and right atria, which receive blood from the venous circulation, and the left and right ventricles, which eject blood into the arterial system (Figure 1.2). The blood enters the right atrium via the superior and inferior vena cavae (the great veins) and moves into the right ventricle, where it is ejected into the pulmonary arteries for circulation through the lungs. On return to the heart, via the pulmonary veins, it enters the left atrium and then the left ventricle, from where it is ejected into the aorta for circulation through the systemic arteries.
The atria are relatively small and thin-walled, reaching peak pressures of approximately 10 mmHg. They require only a minimal contraction to “top up” the ventricular volume, since most of the blood coming into the atria will directly flow into the ventricles during diastolic filling. The ventricles are divided by a muscular interventricular septum and contract simultaneously. The right ventricle will eject blood into the pulmonary circulation and develop peak pressures of approximately 30 mmHg. On the other hand, the left ventricle will eject blood into the higher-pressure, higher-resistance systemic circulation and will develop pressures of approximately 120 mmHg. To handle such high blood pressure, the muscle wall of the left ventricle is much thicker than that of the right ventricle.
The left ventricle ejects approximately 70 mL during each beat (this is known as the stroke volume) and about 5 L per minute (known as the cardiac output). Cardiac output is the product of stroke volume and heart rate. Stroke volume is dependent on the contractile ability of the heart muscle, as well as the amount of blood in the ventricles at the end of filling (the end-diastolic volume, or EDV). Commonly, the EDV in the left ventricle will be in the range of 110–120 mL. After contraction, the end-systolic volume (or ESV) ranges from 40 to 50 mL. The difference between the EDV and the ESV is the stroke volume. Clinically, the term ejection fraction is often used, which represents the stroke volume as a percentage of the EDV. Normal ejection fractions are approximately 66%–68%.
During exercise, when the sympathetic nervous system is stimulated and epinephrine is released, both the heart rate and the contractility of the heart increase. This results in large increases in both the stroke volume and the cardiac output and a decrease in the EDV. With aerobic training, the heart becomes larger and the wall thickness increases (eccentric hypertrophy). This allows for greater ventricular filling and a stronger contraction. Thus, a greater stroke volume can be achieved at rest. If cardiac demand is constant, then the resting heart rate can decrease and still maintain the required cardiac output.
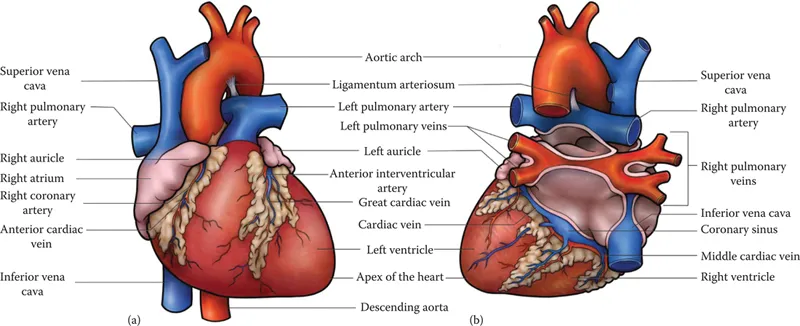
Figure 1.2 (a) The external anatomy of the heart, anterior view. (b) The external anatomy of the heart, posterior view.
1.2 The Heart Valves
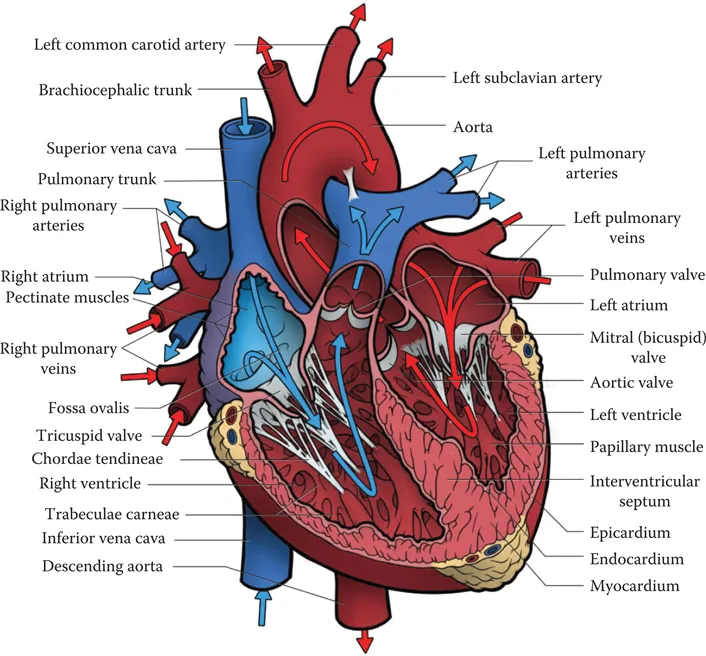
To prevent the backflow of blood, the heart contains two sets of one-way valves (Figure 1.3)—the atrioventricular valves (A-V) and the aortic/pulmonary valves—which are supported by a connective tissue base called the fibrous skeleton. The valves may be open or closed, depending on the pressure difference on either side of the valve. The A-V valves are situated between the atria and the ventricles. The right A-V valve has three cusps (or flaps) and is often referred to as the tricuspid valve. The left A-V valve has two cusps and is often referred to as the mitral valve or the bicuspid valve. When atrial pressure is higher than ventricular pressure during diastole (the relaxation phase), these valves are pushed open to allow for ventricular filling. When the ventricles begin to contract in systole, the increasing pressure in the ventricles pushes the valves closed, thus ensuring only forward blood flow. To prevent the A-V valves from everting, the valves are anchored by small tendon-like fibers called the chordae tendinae, which attach to the valve flaps and originate in the papillary muscle of the ventricles. As the ventricles contract, the papillary muscle also contracts, adding tension to the chordae tendinae and ensuring that the valve remains in a proper closed position. As the ventricles return to relaxation and the ventricular pressure drops below the atrial pressure, the valves open once again.
Figure 1.3 The internal anatomy of the heart, anterior view. Heart valves ensure uni-directional flow of blood through the heart. The atrio-ventricular (A-V) valves separate the atria from the ventricles, labeled as the Mitral valve on the left side and the tricuspid valve on the right. These are anchored by the chordae tendinae and the papillary muscle. The Pulmonary valve separates the right ventricle from the pulmonary artery, and the Aortic valve separates the aorta from the left ventricle.
The aortic and pulmonary valves are found at the base of the heart, where the blood vessels attach. The pulmonary valve separates the right ventricle from the pulmonary artery, while the aortic valve separates the left ventricle from the aorta. During the diastole, the pressure in the blood vessels is higher than in the relaxed ventricles, and the back pressure shuts these valves. With systolic contraction, the pressure in the ventricles will rise. Once it exceeds the vessel pressure, it will push the aortic and pulmonary valves open, allowing for forward ejection of the blood. As diastole returns and the ventricle pressure once again drops below the vessel pressure, these valves will close due to the pressure differential. It is the closing of the valves that can be heard with a stethoscope. The first heart sound, “lub,” is the closing of the A-V valves, while the second heart sound, “dup,” is associated with the closure of the aortic and pulmonary valves.
The A-V valves and the aortic/pulmonary valves are never open at the same time. During diastolic filling, the A-V valves will be open and the aortic/pulmonary will be closed. During ejection, the aortic and pulmonary valves will be open but the A-V valves will be closed. There are two additional heart cycle phases—the isovolumic periods—when all four valves are shut. Because the atria are low-pressure chambers, the A-V valve shuts at the beginning of contraction and the pressure in the ventricle starts increasing. The A-V valve closure marks the beginning of systole. The aorta and the pulmonary arteries are relatively high-pressure systems, and the pressure in the ventricles must overcome this high pressure before they can push the valves open to eject blood. Thus, there is a period in contraction when the A-V valves have shut but the aortic/pulmonary valves are yet to open, and a similar period in relaxation, when the aortic/pulmonary valves have shut but the A-V valves are yet to open.
Heart murmurs are usually a result of a valve abnormality. A valve stenosis refers to a faulty opening of the valve and can be a result of stiff valve flaps or a narrowing of the valve annulus. This may be a congenital defect, caused by an illness such as rheumatic fever. With aging, calcification of the valve flaps also results in stenosis. A valve insufficiency (or regurgitation) refers to a faulty closure of the valve that allows for the backflow of blood. This “leak” can also be a result of a congenital condition, disease, or aging. Both conditions result in less forward blood flow and a drop in stroke volume and cardiac output, which can lead to heart failure. Defective valves may either be replaced with a valve prosthesis—a mechanical valve or a pig or cow valve—or be repaired. These aspects, along with the structure and function of the aortic and mitral valves, will be discussed in more detail in Chapters 9 and 10. Tissue engineering may soon allow for the development of valves from a patient’s own cells.
There are no valves between the atria and the veins that deliver blood to these chambers. With the low pressures created in atrial contraction, the backflow into the veins is minimal.
1.3 Electrical Activity of the Heart
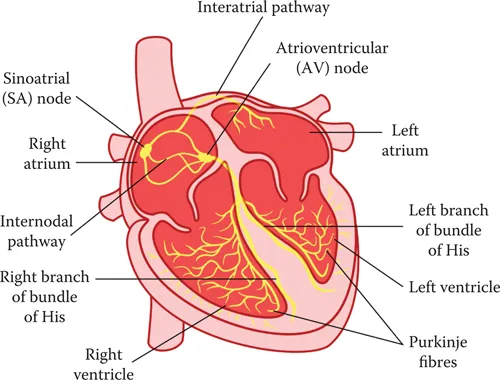
There are two types of cardiac muscle cells—the contractile cells, which create the muscle force, and the autorhythmic cells, which include the pacemaker cells and the conducting system (Figure 1.4). The heart requires no outside signal to contract. The action potential or nervous impulse is initiated in the pacemaker regions of the heart (the sinoatrial or the S-A node and the A-V node). It is then spread through the specialized conducting system (the bundle of His and the Purkinje fibers, which can also initiate action potentials). Each of these pacemaker regions depolarize themselves at different rates, with the S-A node exciting at a rate of 70 beats per minute, the A-V node exciting at a rate of 40 beats per minute, and the bundle of His and Purkinje fibers exciting at a rate of 20–30 beats per minute. Under normal circumstances, the action potential is initiated in the right atrium, by the S-A node, which has the fastest rate of pacemaker cycling (or action potential depolarization). As the impulse spreads to the other pacemaker regions, it causes them to excite before they have a chance to depolarize themselves. Thus, the heart rate (typically 70 beats per minute) is controlled by the S-A node. If this node stopped functioning, the A-V node (the next fastest depolarizing rate) would then control the heart rate (which would drop to about 40 beats per minute). At rest, the parasympathetic system is dominant and acts to suppress the heart rate (from an intrinsic rate of approximately 100 beats per minute, down to about 70 beats per minute). During exercise, when the sympathetic system is dominant, the heart rate increases and the conduction time is quickened.
Figure 1.4 The conducting pathway of the heart. Electrical impulses are usually initiated in the pacemaker region called the sino-atrial (SA Node). It then spreads through the atria to initiate an atrial contraction. It also travels though the intermodal pathway to the Atrioventricular (AV) Node, to the Bundle of His, into the bundle branches and finally out to the Purkinje Fiber system, where the impulse initiates a ventricular contraction.
Once the action potential is initiated in the S-A node, it spreads quickly across the atria via the intermodal pathway towards the A-V node and via the intra-atrial pathway and gap junctions to the left atrium (this takes about 30 ms). This ensures that all the atrial cells are depolarized at the same time and the resulting atrial contraction is unified. The impulse experiences a delay of approximately 100 ms at the A-V node. This delay en...