
eBook - ePub
Principles of Extractive Metallurgy
Fathi Habashi
This is a test
- 494 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Principles of Extractive Metallurgy
Fathi Habashi
Book details
Book preview
Table of contents
Citations
About This Book
Principles of Extractive Metallurgy was planned in four volumes as follows: Volume 1: General Principles, Volume 2: Hydrometallurgy, Volume 3: Pyrometallurgy, and Volume 4: Electrometallurgy. Volume 1was published in 1969 and is concerned mainly with metallurgical kinetics. Divided into 5 sections, this book explores the scope of pyrometallurgy and pollution problems; the engineering aspects especially on heat transfers; the different processes of preliminary treatment of ores; metal separation by reduction, conversion and other processes; and finally refining processes.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Principles of Extractive Metallurgy by Fathi Habashi in PDF and/or ePUB format, as well as other popular books in Technik & Maschinenbau & Werkstoffwissenschaft. We have over one million books available in our catalogue for you to explore.
Information
PART ONE
General
CHAPTER ONE
Scope
INTRODUCTION
Formation of immiscible molten layers
Slags
ENGINEERING ASPECTS
Heat transfer
Compaction of powders
Solid-gas separation
Oxidation of a solid phase
Oxidation in molten phase
Metallothermic reactions
CHEMICAL ASPECTS
Preliminary treatment of ores
Meta1 separation
Refining
INTRODUCTION
PYROMETALLURGY IS that branch of extractive metallurgy which deals with the extraction of metals from their ores by thermal methods. Because of the extensive use of carbon as a fuel and as a reducing agent, pyrometallurgy can be called the technology of carbon, in analogy to organic chemistry, the chemistry of carbon. More than 50% of the coal mined today is used by the metallurgical industry. Pyrometallurgy is the most important division of extractive metallurgy, since it is involved in the recovery of most metals. It is conveniently studied from two points of view:
(1) The chemical aspects and
(2) The engineering aspects.
Before discussing these aspects, it would be advantageous first to point out an important phenomenon in pyrometallurgy, namely, the formation of immiscible molten layers.
Formation of immiscible molten layers
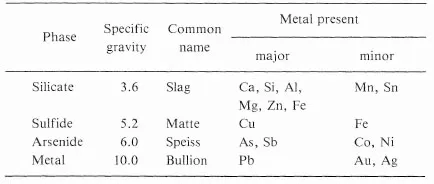
During the melting of an ore or a concentrate, the silicates, sulfides, arsenides, and metals produced coalesce, each forming an individual molten layer. Because of the differences in specific gravity, it is usually possible to allow the material to settle and to separate each layer. The silicate layer on the top is called slag, the sulfide layer next is called matte, and the arsenide layer next, is called speiss. Table 1-1 gives the properties of the different layers formed when melting lead oxide (obtained by the oxidation of a lead sulfide concentrate) in the presence of carbon to get impure metallic lead called bullion. The separation is, however, not sharp; each layer will contain some impurities from the other layers in contact with it. Not all four layers are necessily formed in a process. Most common are the slag-matte or slag-metal layer that form. The case of lead is a particular example.
Table 1-1. Immiscible molten layers formed during the production of lead
Hot gases leaving the furnace are loaded with dust particles. These are mainly components of the charge as well as volatile components formed during melting, e.g., cadmium and indium. In a pyrometallurgical plant, provision is always made to separate the dust from the gas for recovery and to avoid pollution of the environment.
To facilitate the melting process, a flux is usually added to combine with the high-melting point components and form the slag having a low melting point. For example, an ore containing limestone as gangue mineral when heated will result in the formation of CaO which has a melting point of 2580°C. To melt such material, a large amount of fuel will be consumed to achieve this temperature. This approach is not only uneconomical but also will necessitate the construction of a furnace that can withstand such high temperatures. If, however, a flux such as silica (melting point 1728°C) is added to the ore, then on heating, a reaction between CaO and SiO2 will take place to form calcium silicate, i.e., a slag whose melting point may be as low as 1500°C. In this way the gangue minerals are separated from the other valuable ore components in the form of a low-melting slag.
If on the other hand, the gangue mineral was SiO2, then CaO should be added as a flux to aid in the formation of the slag. Fig. 1-1 shows the phase diagram SiO2—CaO.
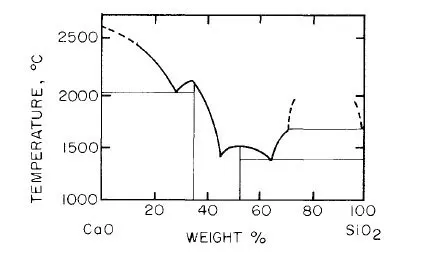
Fig. 1-1. Simplified phase diagram of SiO2—CaO system.
Slags
In pyrometallurgy, SiO2 is considered an acidic oxide and CaO as basic oxide. This concept was introduced long ago when it was observed that some oxides dissolve in water forming an acid, and others when dissolved form a base*. Although SiO2 is insoluble in water, it was regarded as the anhydride of various silicic acids: ortho H4SiO4, meta H2SiO3, and poly H4SiO8, in which the hydrogen atoms can be replaced by metals to form silicates. This is true for a limited number of silicates such as those of the alkali metal group. Recent research, however, has shown that the structure of other silicates is not so simple. Thus, it was found that SiO2 is composed of a large network in which the fundamental structural units is the tetrachedral group SiO4; the central silicon ion is linked with four oxygen ions as shown in Fig. 1-2 and 1-3. In the molten state the network is slightly distored.
In terms of recent theories, and oxide, MO is called a basic oxide because it provides oxygen ions
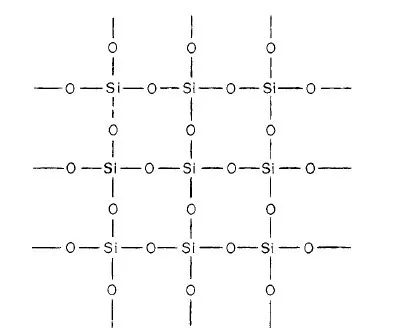
Fig. 1-2. Two-dimensional representation of the structure of SiO2.
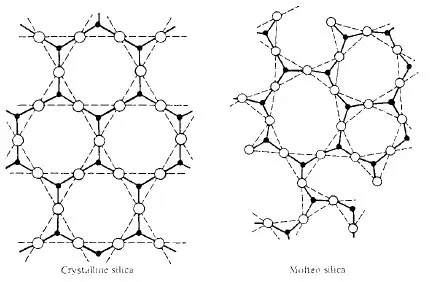
Fig. 1-3. Three dimensional representation of the structure of SiO2.
● Si4+
○ O2−
An acidic oxide, on the other hand, will absorb ions provided by a basic oxide, e.g.:
Figs. 1-4 and 1-5 represent the reaction of CaO with SiO2 to form calcium silicate slag. It can be seen that when CaO dissolves in molten silica, the SiO bonds are steadily broken down, until eventually the melt is composed of discrete groups and Ca2+ ions. A slag, therefore, is a silicate network of various composition. It is considered an acid slag when it is rich in silica, and basic slag when poor in silica, i.e., rich in basic oxides. An acid slag will be capable of dissolving basic oxides while a basic slag, on the other hand, will be capable of dissolving acidic oxides.
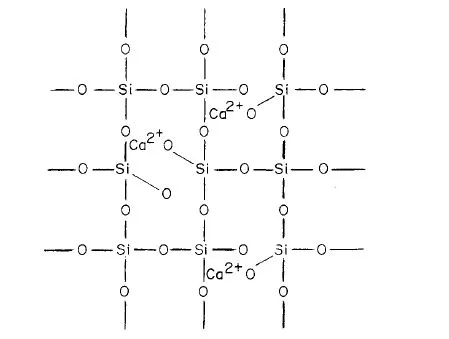
Fig. 1-4. TWo dimensional representation of cal...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Preface
- A Note on Terminology
- PART ONE GENERAL
- PART TWO ENGINEERING ASPECTS
- PART THREE PRELIMINARY TREATMENT
- PART FOUR METAL SEPARATION
- PART FIVE REFINING
- SELECTED REFERENCES
- ACKNOWLEDGEMENTS
- INDEX