
eBook - ePub
Chemical Engineering of Polymers
Production of Functional and Flexible Materials
This is a test
- 458 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Chemical Engineering of Polymers
Production of Functional and Flexible Materials
Book details
Book preview
Table of contents
Citations
About This Book
In this important volume, the structures and functions of these advanced polymer and composite systems are evaluated with respect to improved or novel performance, and the potential implications of those developments for the future of polymer-based composites and multifunctional materials are discussed. It focuses exclusively on the latest research related to polymer and composite materials, especially new trends in frontal polymerization and copolymerization synthesis, functionalization of polymers, physical properties, and hybrid systems. Several chapters are devoted to composites and nanocomposites.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Chemical Engineering of Polymers by Omari V. Mukbaniani, Marc J. M. Abadie, Tamara Tatrishvili, Omari V. Mukbaniani, Marc J. M. Abadie, Tamara Tatrishvili in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Science General. We have over one million books available in our catalogue for you to explore.
Information
PART I
SYNTHESIS AND APPLICATION
CHAPTER 1
COPOLYMERIZATION OF ANILINE WITH P-PHENYLENEDIAMINE IN AN ACETIC ACID MEDIUM
CONTENTS
Abstract
1.1. Introduction
1.2. Experimental Part
1.3. Results and Discussion
Acknowledgments
Keywords
References
ABSTRACT
The oxidative copolymerization of aniline with p-phenylenediamine in an acetic acid medium has been investigated for the first time. It has been determined that as a result of copolymerization; a polymer having a structure analogous to the polyaniline called emeraldine is formed. The obtained copolymer doped with 3N hydrochloric acid has an electrical conductivity five times higher than that of polyaniline prepared by the usual method. Aniline polymerization proceeds more slowly at 273 K under the same copolymerization conditions and the obtained polymer has low conductivity. However, in a mixture of acetic acid-methanol, the reaction proceeds faster and the obtained polymer has conductivity almost equal to that of emeraldine. It has been observed that the conductivity of polymers doped with formic acid is lower than that of polymers obtained by doping with hydrochloric acid by 2 orders of magnitude.
1.1. INTRODUCTION
Among the electro active polymers, the emeraldine form of polyaniline (PANI) attracts intense interest [1,2]. PANI occupies a particular place among the electro active conjugated polymers owing to its environmental stability, easy and cheap method of preparation and its unique properties. It is extensively used in various fields of technology [3–14]. To improve the properties of PANI, studies of aniline copolymerization with different monomers have been conducted [12–19]; however, the conductivity of the obtained copolymers is inferior to that of PANI in spite of high solubility.
According to the literature data, copolymer Ι[20] is formed during the copolymerization of p-phenylenediamine (PPDA) with aniline.
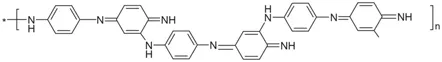
COPOLYMER I
In the formation of copolymer Ι, for 1 mole each of aniline and PPDA, 3 moles of peroxydisulfate (PDS) are required [20] because when using 1 mole of PDS for 1 mole of aniline, the calculated yield for aniline cannot be higher than 33%, while a yield of 62% to 75% was actually obtained [20]. It may be concluded from these data that the reaction does not proceed according to Figure 4 given in the Ref. [20].
According to other literature data, in the case of a molar ratio of aniline/ PPDA of 50:1, the presence of PPDA greatly increases the rate of polymerization and does not affect the structure and crystalline of PANI [21].
These data strengthen our opinion that the reaction mechanism differs from that described in the literature [20], and considering the scheme for the oxidative polymerization of PPDA [22]; we have proposed that it proceeds with a mechanism similar to that depicted in Scheme 1[23].
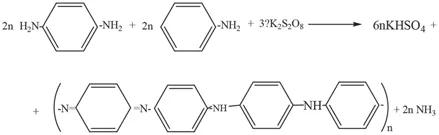
SCHEME 1
If the reaction occurs according to Scheme 1, the maximum yield calculated for aniline will be 67%, while in the case of a ratio of PPDA-aniline in the copolymer of more than 1; the yield will be above 67%.
The purpose of the present work is to verify the possibility that the reaction proceeds according to Scheme 1.
1.2. EXPERIMENTAL PART
1.2.1. MEASUREMENTS
The inherent viscosity of DMSO solution was determined at 25°C, using Ubbelohde viscometer.
The UV/vis spectra of the polymer samples were recorded in 1 cm quartz curettes with Secord 65 spectrometer. FT IR Nicolet Nexus spectrometer was served for obtaining FT-IR spectra in the range of 5000–600 cm−1(KBr pellets). 1H-NMR spectra were obtained in deutera teddi methyl sulfoxide using Mercury 300 Varian NMR spectrometer.
Electrical conductivity was measured on the preliminarily prepared pellets by two-probe method using Teraohmmeter E6–137.
1.2.2. MATERIALS
PPDA was purified by sublimation (mp 416–418 K) and PPDA sulfate was recrystallized from water. Aniline was used after double-distillation. All other chemicals were of analytical grade and were used as received without any further purification
1.2.3. OXIDATIVE COPOLYMERIZATION OF PPHDA WITH ANILINE BY POTASSIUM PEROXYDISULFATE
- Potassium Peroxydisulfate in the hydrochloric acid medium [ 20 ]
To 1.08 g (10 mmol) of PPhDA and 0.91 ml (10 mmol) of aniline in 100 ml (0.1N) of hydrochloric acid, solution of 2.7 g (10 mmol) potassium peroxydisulfate in 190 ml (0.1N) of hydrochloric acid has been added within 3 hours under continuous magnetic stirring at (273–275 K). The solution was kept in refrigerator (270 K) at night. Then, the solution was filtered and the precipitate was washed with distilled water until neutral pH. 190 ml of 0.1 N solution of ammonium hydroxide was added on the precipitate and the mixture was filtered after 13 hour stirring. The precipitate was washed with distilled water until neutral pH and absence of SO4– 2ions, dried in vacuum(323 K/2 kPa) and stored in desiccators over phosphorus pentoxide.The yield is 1.02g (60%).After getting rid of water from the filtrate the ammonia was determined.
- Potassium Peroxydisulfate in the glacial acetic acid medium
- Copolymer (series C-I). To a solution of 0.43 g (4.6 mmol) aniline, 0.93 g (4.3 mmol) PPDA sulfate in 8 ml acetic acid. 1.44 g (6.45 mmol) potassium per sulfate was added under contentious magnetic stirring at 27 K, the mixture was stirred for 3.3 h. After that, the reaction mixture was kept in refrigerator at 270 K. Then the cooled water was added into the mixture. Subsequent procedure was similar to the mentioned above. The yield is 0.42 g (51%).
-
- Copolymer (series C-II). The oxidative copolymerization was carried out by the above-mentioned procedure with the difference in the duration of the reaction (see Table 1.1).
- Copolymer (series C-III). The oxidative copolymerization was carried out by the above-mentioned procedure using acetic acid (8 ml)-methanol (0.8 ml) medium (Table 1.1).
1.2.4. SEMI-QUANTITATIVE DETERMINATION OF AMMONIA
Ammonia was determined using distillation apparatus equipped with liquid trap, receiver filled with 120 ml 0.1N hydrochloric acid for ammonia absorption and Tishchenko bottle filled with water.
TABLE 1.1 Data for Yields, Specific Viscosities and Electrical Conductivities of Aniline Homopolymers (P) and Copolymers (C) with PPDA
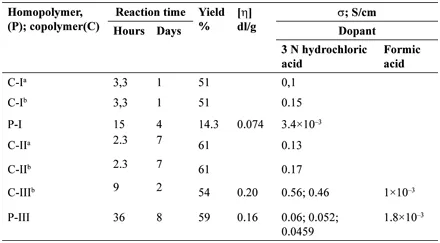
On a dry precipitate obtained after evaporation of the liquid from the filtrate, solution of 2.4 g of KOH in 6 ml of water was added through the dr...
Table of contents
- Cover
- Halftitle
- Title
- Copyright
- Contents
- List of Contributors
- List of Abbreviations
- About the Editors
- Preface
- PART I Synthesis and Application
- PART II Composites and Nanoparticles
- PART III Materials and Properties
- PART IV Green Chemistry and Recycling
- Index