
This is a test
- 144 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
First published in 1985. CRC Press is an imprint of Taylor & Francis.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Crustacean Issues 3 by Adrian M. Wenner in PDF and/or ePUB format, as well as other popular books in Ciencias biológicas & Biología marina. We have over one million books available in our catalogue for you to explore.
Information
Biology Division, Oak Ridge National Laboratory, Tennessee, USA
NCI, National Institutes of Health, Bethesda, Maryland, USA;
Cancer Research Center, Massachusetts Institute of Technology, Cambridge, USA;
Duke University Medical Center, Durham, North Carolina, USA
CONTROL OF MOLTING IN CRUSTACEA
ABSTRACT
The crustacean life cycle, seemingly static for long intervals when the animal is encased in a rigid exoskeleton, is punctuated by proecdysial periods. The single, overriding event that occurs during all proecdysial periods is the synthesis of a new exoskeleton that encompasses an enlarged animal when the old shell is cast off. Regeneration of missing appendages and larval or puberty metamorphoses also occur during proecdysis; all other proecdysial events are directed toward the completion of a new exoskeleton and extrication of the animal from the remnants of the old exoskeleton. Proecdysial periods have been divided into substages defined by the occurrence of specific events. Although a number of factors must be postulated to account for the numerous ‘on’ and ‘off switches of the overall cycle or of individual proecdysial events, only the molting hormone, 20-hydroxyecdysone, has been identified and isolated. Much evidence indicates that the X-organ sinus gland complex, a neurosecretory tissue located in the eyestalks, is the source of a molt inhibiting hormone (MIH) responsible for maintaining animals in anecdysis. An exuviation factor has been proposed to support the extrication of the animal from the old exoskeleton. Additional factors are required to account for other molt-associated phenomena. There is evidence for a limb growth inhibiting factor (LGIF) that affects the rate of growth of regenerating limbs. In addition, we have proposed an anecdysial limb autotomy factor (LAFan) that propels into precocious molts anecdysial animals from which a large number of limbs has been removed and a proecdysial limb autotomy factor (LAFpro) that interrupts the proecdysial period of animals that lose one or more normal or partially regenerated pereopods before a critical time in proecdysis.
1 INTRODUCTION
The simplest description of the control of the crustacean molt cycle requires a molt inhibiting hormone (MIH), the molt stimulating hormone, 20-OH-ecdysone, and an exuviation factor. The interactions of regenerative processes with molting preparations require the postulation of at least three additional factors, either endocrine or nervous.
Included here are brief descriptions of the stages of the crustacean molt cycle, the events that are controlled, and what is known about the hormones and/or other factors involved that exert the controls. Some important enigmas are also mentioned; consideration of the latter may offer new insights and approaches, both intellectual and experimental, to the problem.
2 MOLT CYCLE STAGES
2.1 Overall view of the molt cycle
Starting immediately after the shedding of the old exoskeleton or ecdysis (Stage E), the crustacean molt cycle is subdivided into five major stages and several substages (Drach 1939). Briefly, Stage A, the first stage of metecdysis (Carlisle & Dohrn 1953) immediately follows ecdysis. Stage B is delineated by the initiation of synthesis of endocuticle beneath the epi-and exocuticle layers of the new exoskeleton that were synthesized during proecdysis. Endocuticle synthesis continues through Stage C. An animal is considered to have entered anecdysis (Stage C4) when the membranous layer, the innermost layer of the exoskeleton, has been completed. The next proecdysial period (Stage Do) is signaled externally when the papillae, located at the autotomy plane of limbs lost during anecdysis, initiate regenerative growth (Bliss 1956), and internally when gastrolith formation commences (Travis 1960, McWhinnie 1962). The interrelated events of the ensuing proecdysis that culminate in the next ecdysis are the most thoroughly investigated phenomena of the entire molt cycle.
2.2 Major proecdysial events
2.2.1 Exoskeleton: degradation of old, synthesis of new
The major events that take place as a crustacean enters a period of growth are the synthesis of the epi- and exocuticles of a new exoskeleton beneath the old exoskeleton even as the latter is being degraded. Prior to these occurrences in the integument, the epidermis separates from the old exoskeleton, a process described as apolysis (Jenkin 1966). The occurrence of apolysis marks the initiation of stage D1 (Skinner 1962). During Stages D1 through D3, the membranous layer and most of the endocuticle of the old exoskeleton are degraded, leaving the late proecdysial animal encased in a paper thin, friable shell. Little is known about the enzymes involved in degradation of the exoskeleton.
2.2.2 Muscle: atrophy of fully-formed chela muscle and simultaneous synthesis of muscle in regenerating limbs
These events are described in detail in the chapter by Mykles & Skinner (this volume). Briefly, there is atrophy of approximately 40% of the muscle in chelae (Skinner 1966) but not walking legs (Mykles & Skinner 1982a). A proteinase with the properties of lysosomal cathepsin D was found to increase during proecdysis (Yamaoka & Skinner 1975). More recently, a calcium activated neutral proteinase (CDP) has been purified from crab claw muscle; its activity doubles during proecdysis. This enzyme has the especially interesting property of degrading muscle proteins in preference to non-muscle proteins, i.e. those found in the hemolymph (Mykles & Skinner 1982a,b, 1983). In addition to the loss of both thick and thin myofilaments, in the remaining muscle there is a greater loss of actin and other proteins that form the thin filaments (Mykles & Skinner 1981,1982b). In contrast to the atrophy of chelae muscle (Mykles & Skinner 1982a,b, 1983, 1984), there is concurrent net synthesis of muscle in regenerating limbs (Mykles & Skinner 1982b). Clearly, more than a single factor dictates whether muscle is gained or lost at a particular location.
3 FACTORS
The panoply of molt cycle correlated events and the pattern of their occurrence require at least six hormones and/or other factors that either promote or retard preparations for ecdysis and exuviation itself.
3.1 Molt inhibiting hormone (MIH)
One of the earliest hormones deduced in any species including mammals was a molt inhibiting factor in Crustacea. This factor keeps animals in anecdysis. Although the activity of MIH has been known since the beginning of the century, when precocious molts were observed in several species of Crustacea from which eyestalks were removed (Zeleny 1905), the structure of the MIH and its mechanism of action have not been defined. It has been suggested that inhibition of proecdysis is brought about by the depression of the synthesis and/or release of ecdysone from Y-organs (Soumoff & O’Connor 1982); whether MIH has a more direct action on the target tissues is not known.
Purification procedures for MIH have included low speed centrifugation of homogenates of eyestalks followed by gel filtration through Sephadex of various pore sizes. Active fractions have been thus recovered from extracts of eyestalks of a number of species of brachyurans and other crustaceans. Assays have included inhibition of molting in eyestalkless Ocypode (Rao 1965), or delay of molting in immature Orchestia (Soyez & Kleinholz 1977). The activity is dialyzable, heat stable, and proteinase sensitive. From these and its gel filtration properties it appears to be a small peptide. Progress in the purification of MIH has been seriously impeded by the paucity of material and the lack of a rapid and sensitive bioassay.
3.2 Molting hormone: 20-hydroxyecdysone
The major positive effector of the crustacean molt cycle is indisputably the polyhydroxylated steroid 20-OH-ecdysone β-ecdysone, ecdysterone, crustecdysone) formed in and released from the Y-organ as α-ecdysone and hydroxylated at the C20 position in other tissues (see section 3.2.4). Whether ecdysteroids other than 20-OH-ecdysone are active is not known. Data that support the role of 20-OH-ecdysone as the molting hormone and the Y-organs as the major source of its immediate precursor, a-ecdysone, derive from several types of observations.
3.2.1 Effects of exogenous ecdysteroids
Animals treated with ecdysteroids during anecdysis or early proecdysis, the latter triggered by eyestalk removal in some cases (Lowe et al. 1968, Flint 1972), undergo abbreviated proecdysial periods or show other responses characteristic of a speeded-up proecdysis such as premature apolysis (see Skinner 1984, for more details). In some species (Carcinus maenas, Echalier 1955, Demeusy 1973; Orconectes limosus, Burghause 1975) but not all (Pachygrapsus marmoratus, Charmantier-Daures et al. 1974, Gecarcinus lateralis, Soumoff & Skinner unpublished observations), Y-organectomy prevents or significantly delays proecdysis. Replacement of Y-organs (Echalier 1955) or injection of ecdysteroids (Maissiat 1970, Maissiat & Legrand 1970, Noulin & Maissiat 1974, Keller & Willig 1976, Charmantier-Daures 1976) reestablishes the molting cycle.
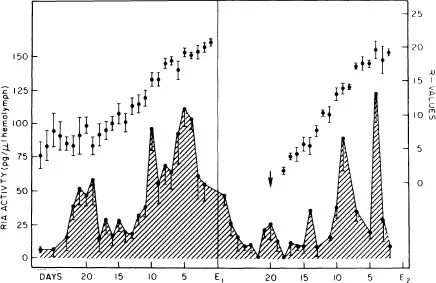
Figure 1. Pattern of daily mean of ecdysteroid titers (ng/ml) in hemolymph of eyestalkless Uca pugilator during two sequential molt cycles (lower curve). Mean daily R values (R = length of limb regenerate (cm) ÷ carapace width (cm) × 100, Bliss 1956) of third limb regenerate (upper curve). Each animal was bled twice on average and each point represents the mean of at least 10 animals. Arrow indicates the point of emergence of limb-regenerates in second proecdysial period. There is high mortality of eyestalkless animals, 50 percent die following first ecdy sis, and another 40 percent in second proecdysial period. Original sample size well over 200 animals. Data from animals that survived first ecdysis plotted in first cycle; data from animals that survived second ecdysis plotted in second cycle. Maximum titer reached early in proecdysial period is one-half that reached later. Pattern does not differ significantly from that of normal animals, except that second peak higher in eyestalkless specimens. Duration of cycle from peak 1 to ecdysis the same in normal and eyestalkless animals (from Hopkins 1983).
3.2.2 Increasing titers of ecdysteroids during proecdysis
Analyses of hemolymph reveal increasing concentrations of ecdysteroids as animals undergo preparations for ecdysis. A maximum peak of ecdysteroid activity is seen in all species during late proecdysis (Stages D2–3; Table 1). An earlier, but smaller peak (peak 1 in Fig.1), reaching approximately 50 % of the level of the major peak, has been seen in some species (Hopkins 1983). It is possible that a similar early peak may be detected in other crustaceans as tissue samples are taken at more closely spaced intervals throughout the molt cycle. The early peak may provide the stimulus for apolysis, initiation of proecdysial growth of limb regenerates, and/or gastrolith formation, all of which occur at or about the same time.
3.2.3 Terminal anecdysis
After the molt to puberty, a number of Crustacea including Callinectes sapidus (Churchill 1918), Maja squinado (Teissier 1935), Libinia emarginata (Hinsch 19...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Foreword
- Preface
- Introduction
- 1 SPECIAL GROWTH TOPICS
- 2 FACTORS INFLUENCING GROWTH PATTERNS
- 3 GROWTH STUDIES IN SELECTED GROUPS
- Taxonomic index
- Subject index