![]()
Part I:
Major RNA Arboviral Zoonoses
![]()
EASTERN ENCEPHALITIS
E. Paul J. Gibbs and Theodore F.Tsai
INTRODUCTION
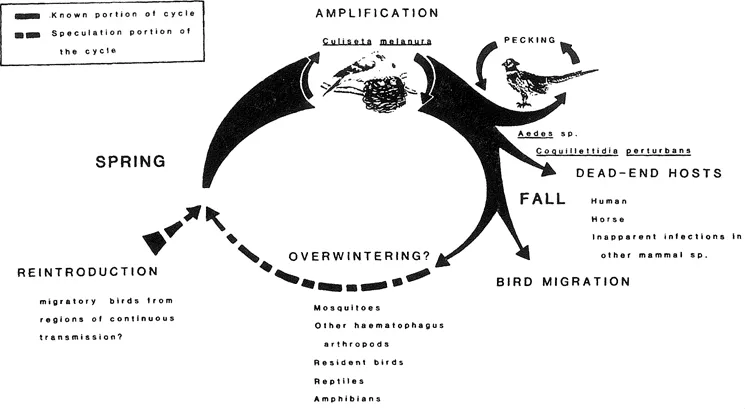
Eastern encephalomyelitis (EE) virus is an arthropod-borne virus classified within the family Togaviridae, genus Alphavirus.11 Since the first isolation of the virus in 1933 from a horse with encephalomyelitis and the differentiation of the virus from Western encephalitis (WE)virus, it has been recognized as the cause of periodic outbreaks of severe disease invariably associated with high mortality rates in horses, humans, and birds exotic to North America. These outbreaks have occurred principally east of the Mississippi River, but outbreaks have been recorded as far west as Texas and on some Caribbean islands. The virus also causes encephalitis in horses in South America. On the continent of North America, the perpetuation of the virus is maintained in a “silent” bird/mosquito cycle (i.e., no clinical disease is apparent) involving passerine birds of freshwater swamps and one species of ornithophilic mosquito, Culiseta melanura (the primary vector or endemic vector) (Figure 1). Several species of secondary-vector or epidemic vector mosquitoes are capable of biologically transmitting the virus to horses, humans, and other vertebrate species in which clinical disease develops. Generally, the level of viremia in these species is insufficiently high to allow further transmission of the virus by mosquitoes; i.e., in evolutionary terms these vertebrate species are dead-end hosts.
Even a superficial analysis of the above statements, within the context of evolution, immediately suggests that EE virus has evolved in North America and established a balanced ecological relationship with the indigenous vertebrate species. Those species that develop clinical disease are recent introductions, in terms of evolution, to North America; human beings are thought to have arrived from Asia approximately 12, 000 years ago with the opening of the Canadian ice corridor, and the modern horse after Columbus.
This concept is supported by recent molecular studies on the evolution of EE virus which suggest that the virus has evolved slowly as a single genetic line in North America. This slow evolution may be due to several factors, but it is most likely a result of the specific adaptation of the virus to a single species of mosquito vector (Culiseta melanura) for its long-term perpetuation.31, 32 It is important to realize that we have accepted this concept of the slow evolution of EE virus in North America (and, by corollary, the failure of the introduced species and EE virus to have coevolved to a successful host-parasite relationship) as the cornerstone for addressing the epidemiology of this fascinating disease.
Despite many years of research on EE by different groups of scientists, many enigmatic features of the epidemiology of this disease remain. The published literature is extensive (see References 15, 17, 23, 29, and 30) and several controversial explanations of the epidemiology of the virus and disease have been proposed.17 There is a wealth of information in the published literature and readers interested in the strict chronology of the literature on EE should consult these reviews. This review focuses on the history and epidemiology of EE in the period since the previous edition of this book in 1981.
The American Committee on Arthropod-Borne Viruses recognized both Eastern encephalitis and Eastern equine encephalomyelitis as acceptable names for the virus and the associated clinical disease.1 Both names are in wide use and should be considered synonymous. However, since the virus can cause disease in several different species, Hayes, 15 the author of the previous edition of this chapter, adopted the shorter terminology of Eastern encephalitis as he considered it less confusing. This nomenclature has been retained in this review. A common lay term for central nervous system (CNS) disorder in horses in North America is sleeping sickness. This aptly describes EE in the horse, but it is also applicable to WE and other nonviral diseases such as hepatoencephalopathy, and is best avoided.
FIGURE 1. The transmission of Eastern encephalitis virus in North America. Although several hypotheses are presented, the overwintering mechanism for EE virus is unknown. (From Scott, T. W. and Weaver, S. C, Adv. Virus Res., 37, 277, 1989. With permission.)
HISTORY
Eastern encephalitis virus probably predates horses or humans in America; it was the likely cause of the first described epidemic of encephalitis in horses in 1831 in Massachusetts. Prior to the isolation of the virus in 1933, extensive outbreaks of what we presume to be EE in horses were recorded on Long Island, New York, in 1845; North Carolina in 1902, New Jersey in 1905, Florida in 1908, and Maryland, New Jersey, and Virginia in 1912.23 Once it had been established in 1933 that epidemics of encephalitis in horses in North America were mostly caused by one of two viruses (EE and WE) that were largely partitioned by geography, more accurate information became available. Epidemics of equine encephalitis in subsequent years established that the principal area of transmission of the EE virus extended from New Hampshire along the Atlantic Coast to the states bordering the Gulf of Mexico. Epidemics in 1942 and 1943 in Michigan demonstrated that EE was not restricted to the coastal states. The largest recorded epidemic of EE occurred in southern Louisiana and adjacent Texas in 1947. Over 14, 000 horses and mules were reported to have been affected, of which nearly 12, 000 died. The fatality rate of 83% in this epidemic is typical of the disease in horses in North America.
With the widespread availability of inactivated vaccines in recent years, epidemics of the magnitude seen in 1947 have been avoided. In the last 25 years the majority of equine cases have occurred in Florida, but small outbreaks have occurred in many of the states east of the Mississippi river. During 1982–1983 it was estimated that there were over 500 cases in Florida and in 1991 there were 159 confirmed cases.20, 33
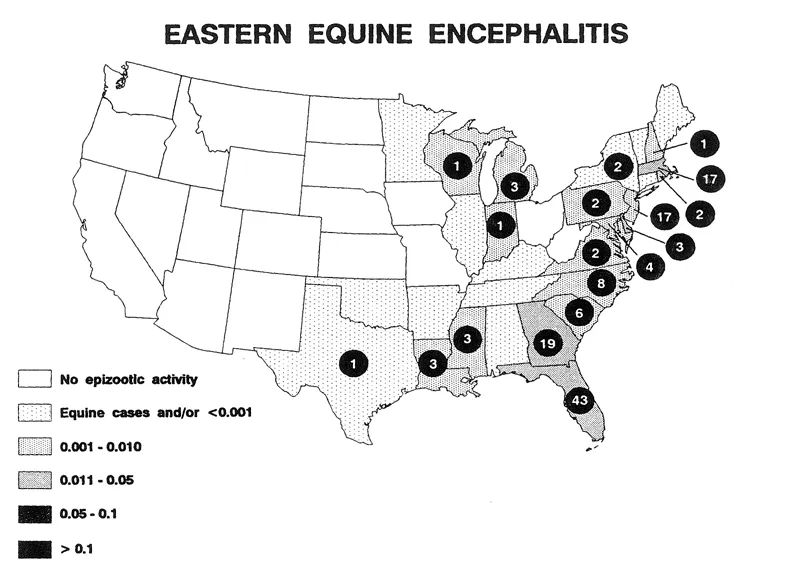
Compared with horse cases, the number of human cases of EE has been small. Other arboviruses that cause encephalitis, such as St. Louis encephalitis (SLE) virus (a flavivirus) are more important when viewed over time and from a national perspective (Figure 2). However, the case fatality rate with EE in humans is high (>30%) and once an epidemic of equine cases is confirmed in a state, the disease causes alarm and has a significant social and economic impact. In the period from 1964 to 1989, 122 confirmed cases occurred in the United States, 38 of which were in Florida (by comparison, during the last major outbreak of St. Louis encephalitis in 1975 there were 2, 800 cases over 31 states). Of the 10 human cases of EE in 1991, 5 were recorded in Florida.5 The annual recognition of EE in horses in several states maintains an awareness in the general public of the virus’ potential to cause human disease.
FIGURE 2. Reported human Eastern encephalitis cases and incidence per 100, 000 by state, United States, 1964–1991.
Eastern encephalitis virus was first shown to cause disease in introduced species of birds in 1937 in ring-necked pheasants in Connecticut. Since that time, disease in sparrows, pigeons, Pekin ducks, and Chukar partridges (all Old World species) has been reported (reviewed by Hayes15). In 1991, EE virus was shown to be the cause of death in emus dying of hemorrhagic colitis in Louisiana;28 similar outbreaks have been reported in emus and ostriches in Georgia, Florida, and Texas in 1992. All the above are nonindigenous species and have been introduced to North America by humans. Similarly, it may be argued that an outbreak of disease caused by EE virus in the endangered whooping crane, kept in a colony at the Patuxent Wildlife Research Center in Maryland reflects the introduction of a non-indigenous species into an area where EE exists.9
ETIOLOGY
Eastern encephalitis virus is classified together with WE and Venezuelan encephalitis (VE) viruses within the alphavirus genus of the family Togaviridae. The alphaviruses are all arthropod transmitted and are found in both the New and Old Worlds; many of the New World viruses cause encephalitis whereas those of the Old World more typically cause fever, rash, and arthralgia. Alphaviruses are spherical, 60–70 nm in diameter, and have an icosahedral capsid covered by a bilayer lipid envelope covered with glycoprotein peplomers; they are not very stable in the environment and are easily inactivated by disinfectants.11 EE virus has a linear, plus sense ssRNA genome about 11, 700 nucleotides in length and the virus replicates within the cytoplasm. Epitopes within the two envelope glycoproteins, El (45–53 K) and E2 (53–59 K), induce neutralizing antibodies whereas antibodies produced to the nucleocapsid protein is broadly reactive across the alphavirus serogroup. Serological and nucleotide sequencing comparisons of the alphaviruses have revealed a complex interrelationship amongst them. Recent studies indicate that EE may have given rise to WE virus through recombination with a Sindbis-like virus from the Old World.14 This recombinant acquired some of the encephalitis-causing potential of EE virus with the antigenic properties of Sindbis virus. The origin of the Sindbis-like virus has not been established and possibly never will be.When this recombination might have occurred is unknown...