![]() Plant Cell Differentiation
Plant Cell Differentiation![]()
12
Cellular Dynamics of the Primary Shoot and Root Meristem
Lam Dai Vu1,2,3,4, and Ive De Smet1,2,*
Introduction
Plants continue to grow during their whole life, and this spectacular growth is largely due to their continuously active stem cells and meristems. In extreme cases, this yields giant redwood trees that are hundreds of years old with active meristems or the trembling giant, a clonal colony of a single male quaking aspen. The necessary primary root and shoot meristems are established early in development – during embryo- genesis – and remain active throughout the plant’s life. In addition, some meristems are established de novo and post-embryonically, such as during lateral root initiation, and these also contribute to the final plant size and shape.
In this chapter, the focus is on the cellular dynamics of the primary shoot and root meristems following their establishment and the various gradients that are present in the meristems. We will highlight the key stages from the birth of a cell over its differentiated stage to – if this occurs – its death, and flag some – but surely not all – key regulatory mechanisms and associated core components involved in regulating and maintaining this. We will mainly focus on data obtained from Arabidopsis thaliana (Arabidopsis) research, and occasionally flag important observations from other plant species.
Early Stages of Embryogenesis Preceding the Establishment of Meristems
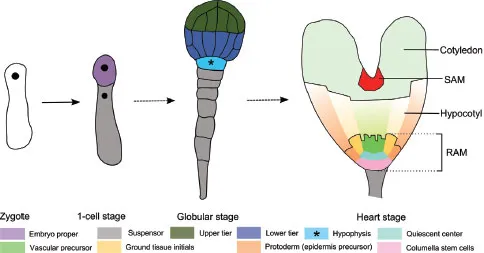
As in any plant developmental process, embryogenesis is largely driven by (oriented) cell divisions (Yoshida et al. 2014). In Arabidopsis, cell divisions occur in a highly ordered sequence which defines the cell patterning in early embryogenesis (Mansfield and Briarty 1991). After fertilization, the zygote loses the polarity that is observed in the egg cell and the nucleus moves to the center of the cell (Ueda et al. 2011, Faure et al. 2002). Before the zygote elongates, the polarity is re-established and subsequently an asymmetric cell division generates a smaller apical cell and a larger basal cell, which mark the apical–basal axis (Mayer et al. 1993, De Smet et al. 2010) (Fig. 1).
FIGURE 1 Four key stages of Arabidopsis embryogenesis. Dashed arrows represent several transition stages in between.
Recently, a 4D map following cell division planes improved our understanding of the geometric framework underlying Arabidopsis early embryogenesis (Yoshida et al. 2014). This 4D map visualizes and demonstrates an auxin-mediated derivation from a default division rule that defines the division plane as the smallest wall area going through the center of the cell, thus allowing asymmetric cell division. These initial, coordinated processes that set up the embryo pattern lie at the basis of primary shoot and root meristem establishment. Several components that play a role in these processes have been identified but precise mechanisms are largely unknown (Lukowitz et al. 2004, Breuninger et al. 2008, Bayer et al. 2009, Jeong et al. 2011, Rademacher et al. 2011, 2012, Ueda et al. 2011, Waki et al. 2011, Yoshida et al. 2014). However, this is outside the scope of this chapter and we therefore refer to some comprehensive reviews (De Smet et al. 2010, Wendrich and Weijers 2013).
Separation of Shoot and Root Domain and Initiation of the First Stem Cells During Embryogenesis
At the globular stage, a pair of closely related leucine-rich repeat receptor-like kinases (LRR-RLK), RECEPTOR-LIKE PROTEIN KINASE1 (RPK1) and TOADSTOOL2 (TOAD2), are required for the maintenance of outer and inner cell separation (Nodine et al. 2007). At this stage, the upper and lower tier of the inner cells begin to express different gene sets. The lower tier divides into the ground tissue precursors and vascular stem cell initials (procambium). This division is marked by the expression of MONOPTEROS (MP) which is important for the specification of the uppermost suspensor cell, called the hypophysis, and cells that form the embryonic root (Berleth and Jürgens 1993, Hardtke and Berleth 1998, Weijers et al. 2006) (Fig. 1). Irregular vascular development is observed in mp mutants, which is caused by defects in polar auxin transport. This is linked to a reduced expression of PIN-FORMED 1 (PIN1), a major auxin efflux carrier (Friml et al. 2003). The MP-promoted PIN1-dependent auxin transport to the hypophysis will activate the auxin response governed by AUXIN RESPONSE FACTORs (ARFs) and AUX/IAA transcriptional repressors, such as ARF9 and IAA10, which are required for hypophysis specification and prevent transformation to embryo identity (Rademacher et al. 2012). Interestingly, the signalling output for hypophysis specification is not directly derived from the primary auxin response but rather adjacent cell-to-cell signalling. This is mediated by the transient interaction of the AUX/IAA protein IAA12/BODENLOS (BDL) with MP (Weijers et al. 2006). Two downstream targets of MP, TARGET OF MONOPTEROS 5 (TMO5) and TMO7, which encode two basic helix-loop-helix (bHLH) transcription factors, are expressed in the lower tier. TMO7 moves from its transcription zone to the hypophysis, where it is necessary to establish a root (Schlereth et al. 2010), while TMO5 has been shown to activate cytokinin biosynthesis (De Rybel et al. 2013, 2014). A mutually inhibitory feedback loop between auxin and cytokinin has been reported to determine post-embryonic vascular pattern in the primary root (Bishopp et al. 2011).
MP activity also controls the expression of PLT1 and PLT2 from the AP2-domain PLETHORA (PLT) family, but not directly (Aida et al. 2004, Schlereth et al. 2010). PLT1 and PLT2 expression is restricted in the lower tier cells at the octant stage and later in the lens-shaped quiescent center (QC) progenitor cell, following the asymmetric division of the hypophysis (Aida et al. 2004). Two other PLT members, PLT3 and PLT4/BABY BOOM (BBM), start to be expressed from the heart stage and accumulate in provascular cells and the lens-shaped cell (Galinha et al. 2007). A severe rootless phenotype was observed in plt1 plt2 plt3 bbm quadruple mutants. Furthermore, TOPLESS (TPL) which is recruited as a co-repressor of several AUX/IAA proteins during embryonic root development (Szemenyei et al. 2008), directly represses PLT1 and PLT2 expression (Smith and Long 2010). Loss of TPL function leads to misexpression of PLT1 and PLT2 in the apical domain of the embryo, resulting in formation of double root seedlings (Smith and Long 2010). This implicates the central role of PLT genes in root apical meristem (RAM) specification and maintenance under control of auxin signalling.
The class III homeodomain-leucine zipper (HD-ZIP III) transcription factor genes PHABULOSA (PHB), PHAVOLUTA (PHV), REVOLUTA (REV) and CORONA (CNA)/ATHB15 (Smith and Long 2010) occupy an expression domain in the upper tier cells at the globular stage under control of microRNA miR165/166 family members (Emery et al. 2003, Mallory et al. 2004). They perform several overlapping, distinct, and antagonistic functions that are important for apical embryo patterning, shoot apical meristem (SAM) initiation and vascular development (Prigge et al. 2005). HD-ZIP III and PLT genes act in an antagonistic manner in controlling apical and basal embryonic cell fate (Smith and Long 2010). Expression of the auxin efflux carrier gene PIN4, which is dependent on PLT1/PLT2 activity, is lost in cells ectopically expressing REV. In addition, gain-of-function mutations of HD-ZIP III genes were shown to repress the double root phenotype in a tpl background. Furthermore, when HD-ZIP III genes are ectopically expressed in the embryonic basal tier, seedlings exhibit a second shoot pole instead of the root pole. This implicates the important role of HD-ZIP III genes in promotion of SAM and antagonism in RAM development (Smith and Long 2010).
The formation and development of the SAM is marked by WUSCHEL (WUS) which is initiated in the upper tier during the globular stage of embryogenesis (Mayer et al. 1998). WUS expression in the embryo is controlled by class III HD-ZIP factors. Simultaneous loss-of-function mutation of the class II HD-ZIP (HD-ZIP II) genes ATHB2 and HAT3, expressed in the early embryo under the direct regulation of REV, reduced the expression of WUS and hence, reducing SAM activity (Turchi et al. 2013). Therefore, these HD-ZIP II genes may play an important role in downstream regulation of SAM development (Brandt et al. 2012, Reinhart et al. 2013).
Maintaining a Developmental Gradient in the Shoot Apical Meristem
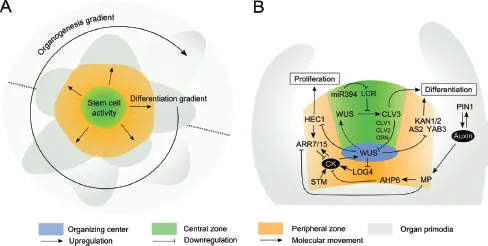
In this section, key aspects associated with the shoot apical meristem will be discussed, and some gradients that exist will be pointed out (Fig. 2A-B). Stem cell proliferation is tightly controlled by the intercellular communication between the organization center (OC) and the central zone (CZ) of the SAM, coordinated by a negative feedback loop, consisting of WUS and CLAVATA3 (CLV3) as the central regulators (Mayer et al. 1998, Fletcher et al. 1999, Schoof et al. 2000) (Fig. 2B). WUS activates stem cell fate in a non-cell autonomous manner, correlated to its migration from the expression zone in the OC to the CZ (Yadav et al. 2011, Daum et al. 2014). The mobility of WUS after expression is highly regulated and promoted by the WUS homeodomain which mediates WUS homodimerization, while being attenuated by a non-conserved sequence between the homeodomain and the WUS-box to prevent WUS from spreading into the peripheral cell and to restrict stem cell induction in the CZ (Daum et al. 2014). Mutations leading to immobilization of WUS in the OC or cell-specific degradation of WUS in the CZ result in loss of stem cells, confirming functional relevance of WUS migration to the CZ. Upon WUS signalling, stem cells in the CZ secrete the peptide CLV3 which represses WUS expression in the OC via several proteins, including the receptor kinase CLV1, the receptor protein CLV2 and the pseudokinase CORYNE (CRN) (Fletcher et al. 1999, Jeong et al. 1999, Brand et al. 2000, Schoof et al. 2000, Müller et al. 2008). In maize, the stem cell-restrictive signal is further transmitted by COMPACT PLANT2, an α-subunit of a heterotrimeric GTP binding protein (Bommert et al. 2013).
The SAM activity and cytokinin signalling pathways are linked by the repressive activity of WUS towards ARABIDOPSIS RESPONSE REGULATOR (ARR) genes, such as ARR5, ARR6, ARR7 and ARR15, which participate in a negative cytokinin regulatory feedback loop (Leibfried et al. 2005) (Fig. 2B). Conversely, plants overexpressing ARR7 have lower WUS RNA level without exhibiting any obvious defects in SAM. However, mutations producing constitutively active ARR7 result in aberrant SAM formation, similar to the phenotype observed in wus mutants. In rice, loss of LONELY GUY (LOG) function cause severe defects in cytokinin biosynthesis and premature termination of the SAM (Kurakawa et al. 2007). In Arabidopsis, the function of LOG genes is more redundant, however, multiple loss-of-function mutations in LOGs result in partially reduced SAM activity. Additionally, LOG4 promoter and cytokinin promoter activity is drastically reduced in clv3 mutants. The coupled antagonistic effects of CLV3 and cytokinin are suggested to control WUS domain dynamics, and there is a negative feedback loop between WUS function and cytokinin biosynthesis (Chickarmane et al. 2012).
FIGURE 2 Developmental regulation in the shoot apical meristem. (A) The main developmental gradients in the shoot apical meristem. The dotted line represents a perpendicular plane illustrated in (B). (B) Key molecular regulators mediate communication between OC, CZ and PZ, CK, cytokinin.
SHOOTMERISTEMLESS (STM), a KNOTTED-1 (KN1) HOMEOBOX (KNOX) family member in Arabidopsis, is expressed throughout the SAM and suppresses cell differentiation by inhibiting the activity of differentiation factor ASYMMETRIC LEAVES 1 (AS1) and downregulating the biosynthesis of the differentiation-promoting hormone gibberellic acid (GA) (Byrne et al. 2000, 2002, Hay et al. 2002). STM also promotes WUS expression by increasing biosynthesis of cytokinin (Hay et al. 2002, Jasinski et al. 2005) (Fig. 2B). Mutants with a reduced cytokinin level in a constitutive GA signalling background are detrimental for SAM function (Jasinski et al. 2005).
MicroRNAs also play a key role in stem cell maintenance in SAM. MiR394, a mobile signal acting downstream of WUS, targets LEAF CURLING RESPONSIVENESS (LCR), encoding an F-Box protein that is suggested to target proteins involved in WUS function (Knauer et al. 2013) (Fig. 2B). In contrast to miR394, the accumulation of miR165/miR166 members leads to loss of stem cell competence in the SAM (Zhou et al. 2007). The sequestration of miR165/miR166 by the ARGONAUTE (AGO) protein AGO10/ZWILLE is essential to prevent the degradation of miR165/miR166 targets, e.g. HD-ZIP III genes, which are expressed in the SAM to maintain it (Zhu et al. 2011, Zhang and Zhang 2012).
The bHLH transcription factor HECATE1 (HEC1) was found to regulate SAM function by promoting stem cell proliferation by uncoupling the WUS-CLV3 feedback loop (Schuster et al. 2014) (Fig. 2B). Its expression level is repressed by WUS and this signal is important for stem cell niche integrity. Enhancing HEC1 activity in the CZ results in a dramatic SAM expansion, while enhanced expression in the OC leads to meristem termination. In addition, HEC1 cell-autonomously activates the expression of ARR7 and ARR15. Furthermore, cell-to-cell movement was observed for ARR7, implying a communication pathway between the OC and CZ and the peripheral zone (PZ) (Schuster et al. 2014). The PZ surrounding the CZ is composed of more rapidly dividing cells that will later differentiate to form lateral organs (Reddy et al. 2004). Non-cell autonomous activity of the WUS-CLV3 pathway has an important function in controlling cell division in the PZ, and thus, defining the boundary between CZ and PZ (Laufs et al. 1998, Reddy and Meyerowitz 2005). Elevated WUS levels or reduction in CLV3 activity induces the expansion of the CZ and at the same time, increase the cell division rate in the PZ, indicatively due to re-specification of PZ cells (Reddy and Meyerowitz 2005, Yadav et al. 2010). Moreover, WUS-mediated transcriptional repression of differentiation-promoting transcription factor genes such as KANADI1 (KAN1), and KAN2, ASYMETRIC LEAVES2 (AS2) and YABBI3 (YAB3) prevents premature differentiation of stem cell progenitors (Yadav et al. 2013).
The formation of lateral organs is initiated when the differentiated cells moves from the PZ tow...