
eBook - ePub
DNA Repair and Replication
Mechanisms and Clinical Significance
This is a test
- 368 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
DNA Repair and Replication
Mechanisms and Clinical Significance
Book details
Book preview
Table of contents
Citations
About This Book
DNA Repair and Replication brings together contributions from active researchers. The first part of this book covers most aspects of the DNA damage response, emphasizing the relationship to replication stress. The second part concentrates on the relevance of this to human disease, with particular focus on both the causes and treatments which make use of DNA Damage Repair (DDR) pathways.
Key Selling Features:
- Chapters written by leading researchers
- Includes description of replication processes, causes of damage, and methods of repair
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access DNA Repair and Replication by Roger J. A. Grand, John J. Reynolds, Roger J. A. Grand, John J. Reynolds in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Genetics & Genomics. We have over one million books available in our catalogue for you to explore.
Information
| Introduction John J. Reynolds, Roger J. A. Grand, and Martin R. Higgs | 1 |
It is now something of a cliché, much used by researchers working in the area of DNA damage and repair, to note that the genome in each human cell is constantly subject to vast quantities of DNA damage, with estimates upwards of 105 lesions per day. However, it is an indication of the effectiveness of cellular DNA repair pathways that, in spite of this continuous assault, almost all cells survive and divide with no long-lasting DNA damage or genome instability. How these repair pathways function and how they are coordinated has been the focus of research for over half a century. The subsequent chapters in this volume will describe the processes surrounding DNA repair in detail, focusing on two closely interlinked repair processes: the cellular response to replication stress and double-strand break repair. The first half of this book will focus more on the mechanisms underlying the DNA replication and repair pathways, whilst the second part will discuss the impact of loss/deficiency of these repair processes on human health and will explore the therapeutic potential of DNA damage and repair research. Indeed, many rare inherited human diseases are attributed to mutations in components of replication and repair pathways, the study of which has been invaluable in understanding the intricacies of the cellular response to DNA damage. To provide a background to these chapters, this short introduction will briefly outline the major forms of DNA damage, the DNA repair pathways which are used to deal with them, and the importance of efficient DNA replication and repair for human health. For the sake of simplicity, references have been omitted here but are included in chapters that form the body of the book.
DNA damage can take many forms, arising from both endogenous and exogenous sources (Figure 1.1), and a multitude of highly conserved, overlapping DNA damage signalling and repair pathways have evolved to deal with any type of DNA lesion that can arise. This coordinated cellular response is collectively called the DNA damage response (DDR). The impact of the DDR on normal cellular functions is reinforced by the fact that a single exposure to ionising radiation triggers in excess of 900 phosphorylation events involving more than 700 proteins, as well as a plethora of other post-translational modifications (PTMs), including SUMOylation, ubiquitylation and methylation. These PTMs play a critical role in regulating the function of many repair proteins, as well as modifying chromatin status, either as part of intracellular signalling cascades or to permit access to repair factors. Chapters 8 and 9, respectively, describe the roles of protein methylation and ubiquitylation/SUMOylation in the DNA damage response. Protein phosphorylation, the most widespread PTM after DNA damage, is discussed, where appropriate, throughout the book.
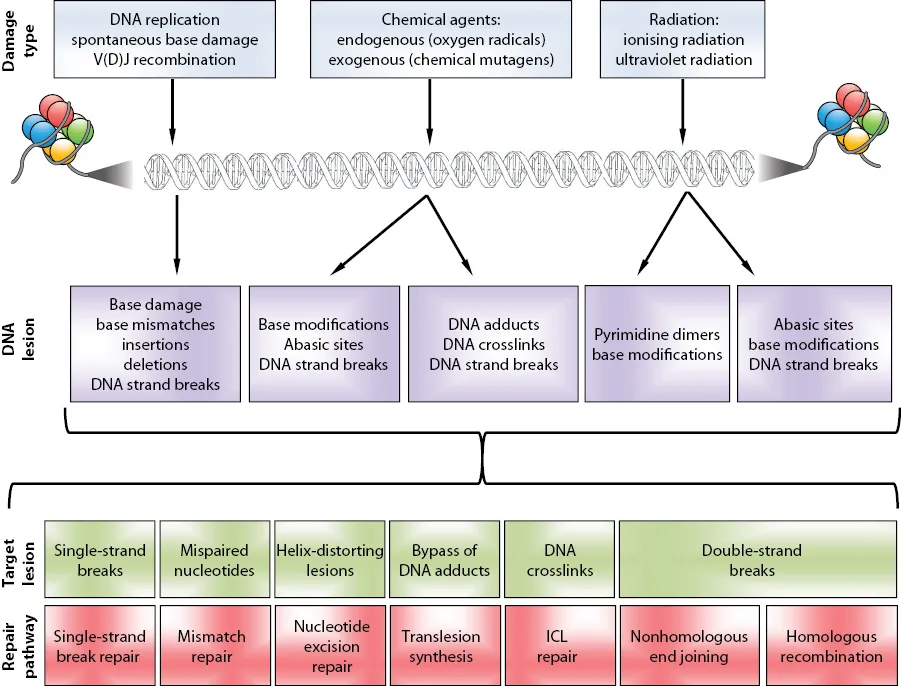
Figure 1.1Diagram showing examples of the major DNA damaging agents (top row), the lesions they cause (second row), the resulting damage to the DNA (third row) and the repair pathways that deal with the damage (bottom row).
DNA double-strand breaks (DSBs) form when both strands of the DNA duplex are broken, and although they are a relatively rare event compared to other forms of damage, they are highly genotoxic if left unrepaired; indeed, it has been shown that a single unrepaired DSB can result in genomic instability, chromosomal rearrangements and/or cell death. DNA DSBs can be caused by exposure to exogenous agents (e.g. ionising radiation), but it is believed that most DSBs arise from endogenous sources, such as the collapse of replication forks following collision with unrepaired DNA lesions during DNA replication, or from the aberrant activity of topoisomerase II. DSBs can be repaired by four interlinked pathways: nonhomologous end joining (NHEJ), alternative NHEJ (alt-NHEJ), homologous recombination (HR) and single-strand annealing (SSA). The choice of pathway used to repair DSBs is dictated by several factors: the phase of cell cycle in which repair takes place, the type of DSB (one-ended versus two-ended), the chromatin status where the DSB is located and the type/structure of DSB lesion. The factors governing pathway choice have received much attention over the last decade.
NHEJ is used to repair the majority of DSBs throughout the cell cycle, and although it can potentially introduce errors and is sometimes considered error-prone, on the whole it tends to be an accurate process. NHEJ is described in detail in Chapter 7 of this book. In comparison, HR is an error-free mechanism for the repair of DSBs and is restricted to the S and G2 phases of the cell cycle to allow the undamaged sister chromatid to be used as a template for strand invasion and DNA repair synthesis. HR, and its particular relevance to DNA replication, is discussed in Chapter 6. Both Alt-NHEJ and SSA provide alternative repair mechanisms to the primary DSB repair pathways (NHEJ and HR) and are also considered in Chapters 6 and 7, respectively. Alt-NHEJ (also known as microhomology-mediated end joining [MMEJ]) makes use of short regions of homology to repair DSBs but, as DNA sequences can be deleted at the repair junction, it is considered error-prone. In contrast, SSA is a subpathway of HR and involves long-range DNA resection of DSBs to uncover homologous DNA sequences. However, unlike canonical HR, there is no strand invasion event as the resected ends are annealed together; therefore SSA is error-prone as it typically results in loss of the genomic regions between homologous DNA sequences.
In contrast to DNA DSBs, DNA single-strand breaks (SSB) are one of the most commonly occurring types of DNA lesion in the cell and account for a large portion of the DNA damage a cell experiences each day. Although an individual SSB is not considered particularly deleterious, unrepaired SSBs can easily be converted into the more genotoxic DSBs upon collision with an ongoing replication fork, and therefore their rapid repair is essential to prevent genome instability. SSBs can arise in several different ways from endogenous sources. They can arise directly from the attack on DNA by reactive oxygen species (ROS) generated by cellular metabolism, indirectly following excision of base damage during the base excision repair (BER) pathway or as the result of abortive enzymatic activity. In all cases, the resulting SSB is repaired by the SSB repair pathway, which is described in Chapter 5.
A significant source of exogenous DNA damage is exposure to ultraviolet (UV) light within sunlight. The most common UV irradiation-induced DNA lesions are cyclic pyrimidine dimers and (6–4) photoproducts which are repaired by the nucleotide excision repair (NER) pathway, described in Chapter 5. Two pathways of NER have been identified, which differ in their mechanism of substrate lesion recognition but use converging repair pathways. Global genome NER (GG-NER) is used to rapidly recognise and repair lesions over the entire genome, whilst transcription-coupled NER (TC-NER) is only activated upon collision of RNA polymerase with a DNA lesion within an actively transcribed gene.
DNA cross-links are DNA lesions in which two DNA bases become covalently linked together, either within the same DNA strand (intrastrand) or between different strands (interstrand), and can arise from both endogenous and exogenous cross-linking agents. Interstrand cross-links (ICLs) are particularly cytotoxic as they pose a severe barrier to DNA replication and transcription, and require the coordinated action of a combination of different protein complexes to repair; of central importance are components of the Fanconi anaemia (FA), HR and translesion synthesis (TLS) pathways. In addition to ICL repair, the FA pathway also performs other vital roles during DNA replication, and is explored in Chapter 12. TLS also functions during DNA replication to promote the bypass of damaged bases by nonreplicative DNA polymerases, and is described in Chapter 4. DNA–protein cross-links (DPCs) are another type of replication blocking lesion that can arise from cross-linking agents, and are recognised and repaired in a recently discovered pathway mediated by the DNA-binding metalloprotease SPRTN, and are discussed alongside the FA pathway in Chapter 12.
Although the different forms of DNA damage discussed above are deleterious to genome integrity in their own right, they are especially important when viewed in the context of the two essential cellular processes that use DNA as a template: DNA replication and transcription. The process of cellular DNA replication is fundamental for the continuity of life, and thus the cell employs numerous tightly regulated mechanisms to ensure it proceeds in a timely and efficient manner. Cell cycle factors operate to ensure that initiation, progression and completion of replication occurs before the event of cell division and that the genome is only replicated once during each cell cycle (described in Chapter 2). Additionally, recent evidence has illustrated that a failure to terminate DNA replication successfully can also give rise to genome instability (detailed in Chapter 3). Furthermore, the cell faces the challenge that all enzymatic processes have a certain error rate, and therefore during the course of DNA replication, DNA polymerases will sometimes incorporate the wrong DNA base or produce insertion–deletion mispairs by polymerase slippage at repetitive DNA sequences. To correct these polymerase errors, and prevent them from being turned into permanent mutations in later rounds of the cell cycle, the DNA mismatch repair (MMR) pathway operates during the S phase to remove and repair any base mismatches or insertion–deletion loops (outlined in Chapter 12).
DNA replication also occurs in the face of numerous obstacles, including repetitive DNA sequences, transcription–replication conflicts, DNA lesions (small and bulky DNA adducts, SSBs, cross-links etc.), DNA-protein cross-links and DNA secondary structure. These all have the potential to impact negatively on the process of genome duplication, giving rise to a cellular state known as ‘replication stress’, which is broadly defined as a state in which DNA replication is impeded, leading to the slowing or stalling of DNA replication forks. It has been suggested that stalled replication forks represent one of the most dangerous lesions that cells encounter, as they will impact on chromosomal duplication and can result in chromosomal rearrangements, cell death or cellular transformation. In this context, it is clear that repair of DNA lesions by the combined actions of NER, SSBR, ICL repair and DSB repair are therefore crucial to maintain successful DNA replication, since forks that encounter these lesions will either stall or collapse to form a one-ended DSB, which requires HR for repair (detailed in Chapter 6). Moreover, prolonged fork stalling can also give rise to replication-associated DSBs. A cellular response to replication stress has therefore evolved to coordinate the processes of DNA replication, cell cycle progression/arrest, DNA repair and replication fork stability/restart, to ensure the successful completion of DNA replication despite the continued threat of DNA damage (described in Chapter 2).
Unrepaired DNA damage also poses a challenge to transcription. Additionally, as DNA and RNA polymerases share the same DNA template, collisions between the replication and transcription machinery also have an impact on transcription. Therefore, the cell employs repair pathways that specifically repair DNA lesions within actively transcribed genes (TC-NER), and uses several strategies/processes to resolve replication–transcription conflicts. The interplay between transcription, replication and DNA repair is addressed in Chapters 10 and 11.
Given all this, it is abundantly clear that the repair of genetic damage is of fundamental importance for the maintenance of genome stability and cellular integrity, and that these pathways have enormous clinical significance. It is therefore unsurprising that a large number of human disorders are caused by mutation or loss of proteins involved in DNA replication and DNA repair pathways. Although these human disorders exhibit a large amount of phenotypic variability, there are common clinical features to many of the diseases, including neurological dysfunction, immunodeficiency, growth retardation, developmental defects and cancer predisposition.
The link between DNA damage and human disease was established for the first time in 1969, when patients with xeroderma pigmentosum (XP), a disease characterised by sensitivity to sunlight and a 1000-fold increased risk of developing skin cancer, were found to be defective for the repair of DNA damage induced by UV light. Mutations in components of the NE...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Contents
- Detailed Contents
- Preface
- Editors
- Contributors
- Chapter 1: Introduction
- Chapter 2: DNA Replication and Cell Cycle Control
- Chapter 3: DNA Replication Termination and Genomic Instability
- Chapter 4: Mechanisms of DNA Damage Tolerance
- Chapter 5: The Repair of DNA Single-Strand Breaks and DNA Adducts: Mechanisms and Links to Human Disease
- Chapter 6: Homologous Recombination at Replication Forks
- Chapter 7: Mechanism of Double-Strand Break Repair by Non-Homologous End Joining
- Chapter 8: Protein Methylation and the DNA Damage Response
- Chapter 9: Ubiquitin, SUMO and the DNA Double-Strand Break Response
- Chapter 10: Transcription in the Context of Genome Stability Maintenance
- Chapter 11: RNA Binding Proteins and the DNA Damage Response
- Chapter 12: DNA Replication and Inherited Human Disease
- Chapter 13: Ataxia Telangiectasia and Ataxia Telangiectasia–Like Disorders
- Chapter 14: DNA Repair Mechanisms in Stem Cells and Implications during Ageing
- Chapter 15: Targeting Replication Stress in Sporadic Tumours
- Chapter 16: A Few of the Many Outstanding Questions
- Index