Nutrients and growth factors regulate brain development during prenatal and postnatal life. Animal studies have demonstrated that brief periods of dietary manipulation, either deficiency (absolute and relative) or supplementation, during a vulnerable period of brain development could have long-lasting effects on the structure and function of the brain (Dobbing, 1990; Lucas, 1994). Although such a programming effect has not been conclusively demonstrated in humans, epidemiological studies suggest that early nutrition may influence neurodevelopment in humans as well (Lucas, 1998). However, due to its plasticity, a developing brain may also be more amenable to repair following such nutritional perturbations (Dobbing, 1990; Morris, Halliwell, & Bowery, 1989). Furthermore, due to the prioritization of nutrient delivery to the brain and the filtering effect of the blood-brain barrier, the developing brain may be spared the adverse effect of nutrient perturbations. Despite such structural brain sparing, nutritional deficiencies may still exert an adverse effect on the functional outcome of neurodevelopment (Georgieff, 1998). For the same regulatory reasons, nutrient overabundance may produce positive, negative, or no effects on the brain. In this chapter, the association between nutrients and early neurodevelopment are discussed. The general principles of research on the interaction between early nutrition and neurodevelopment are discussed initially, followed by a brief discussion on the role of individual nutrients on neurodevelopment. Because of their global significance, two conditions—chronic energy malnutrition and iron deficiency—are reviewed. Finally, the role of nutrient supplementation on neurodevelopment is discussed.
General Principles of Nutrition
All nutrients are essential for normal neuronal cell growth and development. The timing of delivery of the specific nutrient is important in determining its effect on brain development. For example, in rats, iron deficiency during the perinatal period results in a small brain size (Rao et al., 1999), decreased oxidative metabolism in cognitively important areas of the brain (de Ungria et al., 1999), and permanent adverse effects on learning and behavior (Felt & Lozoff, 1996), while iron deficiency in postweanling rats has minimal, if any, effect on brain size (Chen, Conner, & Beard, 1995) and no effect on oxidative metabolism (Mackler, Person, Miller, Inamdar, & Finch, 1978). Furthermore, brain iron deficits in such situations appear to be amenable to iron rehabilitation (Youdim & Ben-Shachar, 1987). Epidemiological studies have suggested that such an effect of timing might also be operational in humans. Although early nutritional restriction during the intrauterine period (e.g., due to placental vascular insufficiency) results in a smaller brain size in infants, late onset intrauterine growth retardation (IUGR) due to pregnancy-induced hypertension in the mother has a brain-sparing effect (Greene, 1991). Similarly, while the adverse cognitive effects associated with early iron deficiency appear to be irreversible (Lozoff, 1990), those present in iron-deficient older infants and children probably could be reversed by iron therapy (Bruner, Joffe, Duggan, Casella, & Brandt, 1996).
A nutrient that promotes normal brain development at one time may be toxic at another. For example, while iron is essential for normal brain development during infancy, abnormal iron homeostasis with demonstrable iron deposition has been postulated in the pathogenesis of such neurodegenerative disorders as Parkinson’s disease (Kienzl et al., 1995) and Alzheimer’s disease in adults (Cornett, Markesbery, & Ehmann, 1998). In addition, a nutrient that promotes normal brain development at a particular concentration may be toxic at another, as seen by the development of microcephaly and mental retardation with exposure to high levels of vitamin A during early pregnancy (Willhite, Hill, & Irving, 1986). Many nutrients are regulated within a narrow therapeutic range.
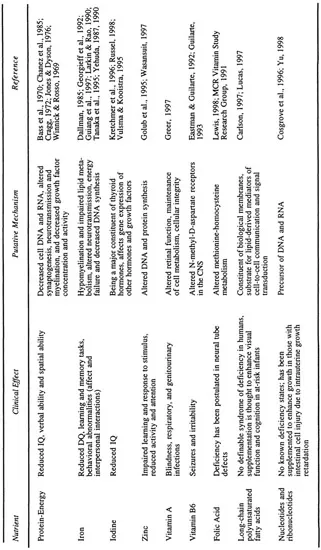
Individual nutrients can affect brain development through their effect on neuroanatomy, neurochemistry, or both. The structural effects are due to the effect of nutrients on cell division and growth. Such an effect on neurons would affect the size of the developing brain, while the effect on supporting structures (e.g., oligodendrocytes) could affect myelination and nerve conduction. Neurochemical effects of nutrients are mediated through neurotransmitter-receptor synthesis and regulation. While malnutrition (deficiency or overabundance) of any of the nutrient categories could adversely affect brain development, certain nutrients appear to exert a more profound effect than others. Depending upon the nutrient and the timing of its malnutrition, these adverse effects could be reversible or permanent. Table 1.1 provides a list of the nutrients and their putative effect on the developing brain.
Just as nutrient deficiencies can have an adverse effect, supplementation of certain nutrients may have a beneficial effect on neurodevelopment. Supplementation of infant formula with certain long-chain polyunsaturated fatty acids (LCPUFAs,
TABLE 1.1 Effect and the Putative Mechanism of Individual Nutrients on Neurodevelopment
discussed later) such as docosahexaenoic acid (DHA) and arachidonic acid (AA) (Carlson, 1997; Innis, 1997; Woltil, van Beusekom, Schaafsma, Muskiet, & Okken, 1998) or nucleotides (Axelsson etal., 1997; Woltil et al., 1995; Yu, 1998), as well as general fortification of preterm breast milk (Gordon, 1997; Lucas, 1998) may confer beneficial neurodevelopmental effects. While studies are essential to evaluate the effect of a particular nutrient on brain development in a controlled manner, it must be remembered that nutrients rarely act in isolation (Lozoff, 1990). The influence of an individual nutrient on brain development depends upon its interaction with other nutrients, the amenability of the developing brain to nutritional intervention, and environmental stimulation (Dobbing, 1990).
Fundamental Questions on the Relationship of Early Nutrition to Neurodevelopment
The importance of early nutrition on neurodevelopment has been the focus of research since the turn of the 20th century (Haiti, 1904) and has gained renewed interest recently (Dobbing, 1990). Initial studies focused on the outcome of children affected by early malnutrition from developing countries and were confounded by other environmental factors, such as lack of stimulation and poor socioeconomic status (Cravioto, de Licardie, & Birch, 1966). More recently, experimental nutritional interventions with strict randomization of groups and adequate follow-up have provided more compelling data that early nutrition affects brain development in humans (Gorman, 1995; Morley, 1996).
Any research on the interaction between early nutrition and brain development should:
- Assess whether nutrient-induced structural alterations also result in functional alterations in the developing brain (e.g., behavior). An effect on brain structure without associated adverse functional outcome may not be of clinical significance (Dobbing, 1990).
- Establish the closeness of the linkage between structural and functional outcome.
- If the two are not closely linked, then explore the reasons for the variations in the functional (i.e., behavioral) outcome of nutritionally based alterations in brain development. If confounding covariables appear responsible for the variations in the functional outcome, the possible usefulness of these covariables in designing remedial measures that would reverse or minimize the adverse effects of malnutrition on neurodevelopment need to be evaluated.
Experimental Approach: Clinical Trials in Humans
To answer these fundamental questions, appropriate experimental methodology is necessary. One approach has been to conduct clinical studies in representative populations. By and large, these have been epidemiological studies of specific nutrient deficiency (with or without subsequent supplementation) in a population at risk (e.g., effect of iron deficiency on learning and behavior by Deinard, Gilbert, Dodds, & Egeland, 1981; Lozoff, Wolf, Urrutia, & Viteri, 1985; Walter, 1994). Epidemiological studies need not be on deficiency states alone. The effects of nutrient supplementation on neurodevelopment have also been studied by this method (Waber et al., 1981). While providing valuable information about the population at risk, the confounding variables that are also present in the populations studied can often result in indirect implications of the role of the nutrient in outcome measures in such studies.
One way to overcome the effect of confounding variables is to conduct randomized clinical trials of individual nutrients. For ethical reasons, these have been mostly supplementation studies, although short-term deprivation studies have also been conducted (Walter, De Andraca, Chadud, & Perales, 1989). Examples of such studies include protein-energy supplementation of infants and children (reviewed by Gorman, 1995), DHA supplementation on cognitive development and visual acuity of preterm infants (Carlson, 1997), and folate supplementation during peri-conception and postconception to prevent neural tube defects (MRC Vitamin Study Research Group, 1991). To overcome the effect of confounding variables, as well as to avoid inferences due to chance alone (beta error), large sample sizes are necessary. Despite having a sufficiently large number of enrollees, a clinical trial may still run the risk of assigning cause and effect to an epiphenomenon (i.e., both factors are true, but unrelated by cause and effect). Also, the data may be specific for the population from which the sample has been drawn and hence cannot be generalized. Finally, clinical studies usually do not provide any information about the mechanism of the interaction between the nutrient in question and neurodevelopment. They are useful to postulate questions about cause and effect, and therefore the putative mechanism. These postulations frequently lead to mechanistically based experimental designs in humans and in animal models.
Small-scale clinical trials have been conducted to evaluate the putative mechanism through which a specific nutrient exerts its effect on neurodevelopment. An example of such a trial is the use of visual evoked response (VER) for assessing the effect of DHA supplementation in human infants (Birch, Hoffman, & Uauy, 1992). VER could be considered appropriate in this instance since it provides a measure of myelination of visual neural tracts, the putative mechanism through which DHA is hypothesized to be beneficial (Neuringer, Connor, & Lin, 1986). Such clinical trials have some shortcomings. The putative mechanism of interaction with neurodevelopment is generally obtained from animal models and may not be extrapolated to humans. Since the human trials evaluate the putative mechanism, there is a danger of assigning a false role (positive or negative) to the nutrient in question. A structural, metabolic, or both types of change in the brain may not imply or predict a related behavioral change in humans and animal models (Dobbing, 1990). Furthermore, the conventional tests used to evaluate the functional outcome in humans may not measure the motivational aspect and the capacity to perform. Finally, the small-scale clinical trials may not have any clinical relevance to the population at large.
In order to link nutrient status to neurodevelopment, large- and small-scale randomized clinical trials in humans must meet a certain standard to constitute a biologic proof of cause and effect. Typically, in nutrient-neurodevelopment research, psychological tests are used as outcome measures. According to Singer (1997), in planning a prospective clinical study, the psychologic assessment must: (a) be standardized (i.e., have a uniform set of procedures for administration and scoring); (b) be reliable (i.e., measure a cohesive construct in a similar fashion across time); and (c) be valid (i.e., measure the construct it purports to). Given the increasing knowledge of cognitive neuroscience [functional MRI (fMRI) and real time event related potentials (ERP)], a fourth criterion must now be considered; that is, the psychological assessment must be matched to the pathophysiology of the biologic event (Georgieff, 1998).
In matching the psychological assessment to the underlying central nervous system (CNS) pathology, most researchers rely on clinical or animal models. Evaluating for signs of spasticity in the lower extremities as an outcome measure of periventricular leukomalacia in preterm infants is an example of such a model. Lower extremities are preferentially affected in that condition due to involvement of the descending motor tracts that subserve the lower extremities (Volpe, 1998). While such motor models appear to be specific and sensitive to the underlying pathology, cognitive models are more difficult to create because of the diffuse nature of cognitive circuitry (Nelson, 1995). Applicability of such models in the evaluation of the cognitive system depends upon the insult and the brain structure vulnerable to injury in such insult. For example, since the hippocampus is targeted in iron deficiency (de Ungria et al., 1999; Erikson, Pinero, Connor, & Beard, 1997), evaluation of recognition memory could be considered a specific test for assessing the effect of iron deficiency on cognitive development. A more generalized test, such as the Bayley Scales of Infant Development, in such a situation probably has less predictive value (Walter, 1990). Conversely, Bayley Scales appear to be more useful for evaluation of the effect of protein-energy malnutrition, which has a more global effect on cognitive systems (Pollitt & Gorman, 1994). Other more complex behaviors (e.g., anxiety) recruit neural generators from across the CNS, and may be very difficult to assess with respect to nutrient involvement.
Experimental Approach: Animal Models
Research has relied heavily on animal models to understand the mechanisms of interaction between nutrition and brain development. Such models allow isolation of individual nutritional variables while controlling for genetic-environmental variables. With careful choice of the species (homology to human brain vis-à-vis structure, timing and nutrient homeostasis) and standardized tests (generally based on the specificity for the anatomic site), models can be created to test various hypotheses of interaction between nutrient(s) and brain d...