
- 520 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Environmental Science
About this book
First published in 1983. This book aims to cover the requirements for the Business and Technician Education Council Level Three & Four Units in Environmental Science. At the same time it was recognised that there was a real need for a suitable book for those studying Building, Quantity Surveying, Architecture and Environmental Health at 1st degree level. This book should therefore form a useful first year introductory text for both 'A' level and BTEC entrants. The book contains a large number of worked examples in the text as well as many student questions at the end of each chapter. Experiments have been included, not with the intention of being exhaustive, but to give ideas. Some areas of work lend themselves to student practical work better than others so that some inbalance is inevitable. 'Environmental Science' should give students an introduction to the environmental problems in construction and the methods which may be used to provide a satisfactory and economic solution.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Heat and thermal effects
Chapter 1
Thermal transmission
Temperature
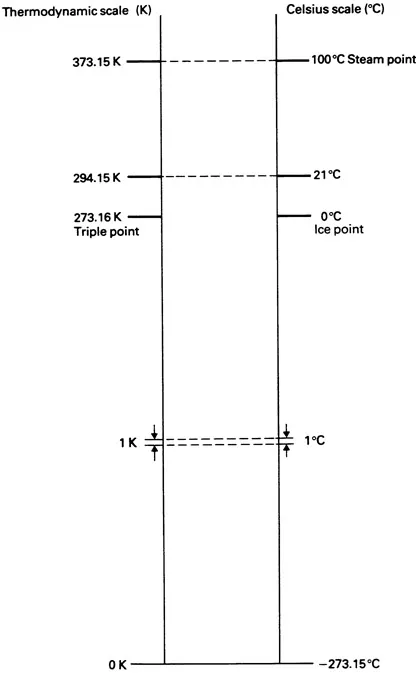
Thermodynamic temperature scale
Celsius temperature scale




Quantity of heat
Sensible heat
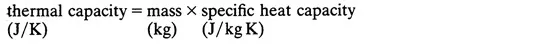

- where, m = mass (kg)
- c = specific heat capacity (J/kg K)
- and θ = temperature difference (K)
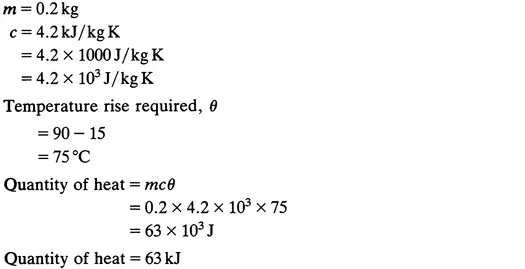
Latent heat
Transmission of heat energy
Conduction
Table of contents
- Cover
- Title
- Copyright
- Contents
- Preface
- Acknowledgements
- List of units
- Section I Heat and thermal effects
- Section II Sound
- Section III Light
- Section IV
- Section V
- Answers
- Bibliography
- Appendix
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app