![]()
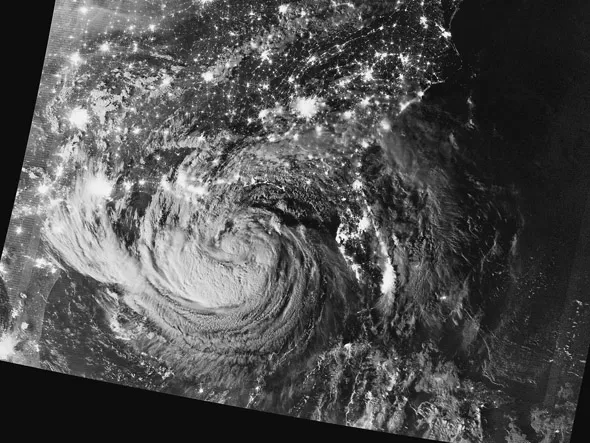
Figure 1.1 Two scales of environmental self-organization, weather, and urban settlements. A nighttime view of Tropical Storm Isaac and the cities near the Gulf Coast of the United States, August 28, 2012
NASA, Visible Infrared Imaging Radiometer Suite (VIIRS) image from Suomi-NPP satellite
Chapter One
Environments of maximum power
We are taking “survival of the fittest” to mean persistence of those forms which can command the greatest useful energy per unit time (power output).
(Odum and Pinkerton 1955, 332)
The reassuring images of a harmonious global environment, from the stable climate zones of the ancient world to NASA’s “blue marble in space,” have little to offer contemporary designers faced with a changing and self-organizing environment (see Figure 1.1). Even the productive agricultural civilizations of the last 10,000 years can be blamed for tipping many of the arid regions of the world into desert. Designers need help thinking about our situation in an endlessly changeable and changing climate, to grasp the interaction between our individual acts of building and the environment as a whole. The natural task of individual buildings, institutions, or economies is to determine the best use of the scarce resources that they compete to obtain, while the biosphere is working to dissipate the steady flow of energetic wealth from the sun and the planet’s core. As Georges Bataille argued, the individual ethics of scarcity are simply different than the collective urgency of abundance, but the two can be linked by tracing the power that fuels them both (Bataille 1988).
We say that individuals “choose” while the environment “selects.” But the environment can only select among things already birthed or built by the individuals that make up the whole. The environment that we encounter is the material result of the many arrangements constructed over time by different species, ecosystems, and human enterprises, each dissipating its share of energy to build for their particular purposes. The slow process of natural selection among species has been overtaken by the rapid adaptation tactics of humanity, which have liberated the power of information from the slow DNA mutations of speciation to the accelerated information cycles of technological innovation. The different rates of those techniques have been destabilizing. Aldo Leopold argued for an environmental approach that linked the two rates of adaptation, a “land ethic” based on a deeper understanding of the slower ecological food chain that the more rapid human enterprises have territorialized (Leopold 1949). A full account of the environment combines the ethics of the part and the whole, of the fast and the slow, in a thermodynamic analysis of the energy, materials, and information with which it is made.
Accounting for a living, self-organizing environment
The architectural profession has struggled to develop forms of environmental accounting that can guide the design process without overly burdening or complicating it. In addition to regulatory codes, a variety of approaches have emerged, which offer expanded forms of assessment: ecological footprints, carbon footprints, embodied energy, life cycle assessment (LCA), cradle-to-cradle, and ecosystem services review all offer enhanced methods for capturing some aspect of the environmental impact of materials, products, or buildings. The most widely recognized environmental standard for buildings is the US Green Building Council’s (USGBC) Leadership in Energy and Environmental Design (LEED) program, which is an independent, “aspirational” standard meant to provide “building owners and operators with a framework for identifying and implementing practical and measurable green building design, construction, operations and maintenance solutions.” (USGBC) LEED largely draws on existing standards—such as the model energy codes developed by the American Society of Heating Refrigerating and Air-conditioning Engineers (ASHRAE) and the EPA’s EnergyStar rating system—and assigns subjective points to reconcile the differences between measurements of energy use, water consumption, or indoor air pollution.
The very variety of codes and standards—and especially the competition among voluntary evaluation systems like LEED, BREEM®, Green Globes, and others—is symptomatic of the lack of a comprehensive science of environmental sustainability. LEED has achieved pragmatic clarity at the expense of a more comprehensive assessment or more ambitious goals. By providing checklists of contemporary best practices, LEED has increased market penetration but does little to foster innovation or the radical change that is needed.
To the USGBC’s credit, one of the most ambitious, aspirational standards has emerged from its own “left wing”—the International Living Future Institute, which administers a performance standard called the Living Building Challenge (LBC). The goal of the LBC is to promote buildings that contribute as productively to their ecosystems as trees and plants. To realize this challenge, the LBC developed a suite of 20 or more performance-based “imperatives” that are instructive both for their environmental ambitions and the limitations they reveal (LBC 2012). The first five categories of the challenge are similar to those used in the LEED program—site, water, energy, health, and materials; two additional categories, equity and beauty, seek to extend the challenge into social and cultural conditions. Taken together, the seven categories capture most of what we would expect in an environmental metric and, within each imperative, the LBC has adopted the most ambitious current approach: net zero energy, net zero water, and a “red list” of prohibited materials. Despite the ambition of the 20 imperatives, however, the LBC provides no objective method for comparing the value of one imperative with another, for assessing the total environmental impact of a building project, or ultimately for optimizing a particular design.
The Living Future Institute is admirably clear that it is setting goals, rather than offering a complete method through the LBC. But the lack of a fundamental environmental value is a problem common to most of the building standards, metrics, and tools currently in use. Broadly speaking, those measures can be divided into two classes: objective and reductionist, and those with some degree of subjective weighting. LEED and the LBC both apply subjective evaluations to the results of reductionist calculations of energy consumption, water use, or embodied carbon, and use professional judgment or current practices to establish the relative importance of the different criteria.
When the Living Building Challenge offers life as a goal for design, it invokes the longstanding use of biological analogies in design—from biotechnics and biotechniques to bionics and biomimicry—and points the way to an ecological form of design based on a deeper understanding of the place of buildings in the environment (Steadman 1979; Mertins 2004). It is telling that the LBC refers to trees and other plants, and not animals, especially not predators, as the model for “living” buildings. The immobility of plants strengthens the analogy, but most buildings are more like herbivores that consume plant products, while some high-powered buildings are more like carnivores or carrion eaters that feed at higher levels or on the detritus of the food chain.
While useful as a starting point, analogies are not entirely sufficient to imagine an ecological form of building for the anthropocene. We need to understand the mechanisms that drive the operation of ecosystems, as well as the ways in which human designs differ from (and resemble) the evolution of species or the self-organization of ecosystems. Over the last century, biology, ecology, and economics have developed new techniques to describe the behavior of self-organizing systems, based on the thermodynamic exchanges of food, energy, money, and information, which can reveal the organization and trajectories of those systems.
Thermodynamics of living systems
Precisely because of its universality, energy can be confusing. It combines common-sense understandings of work with a number of more precise concepts such as heat, exergy, entropy, power, and embodied energy. In the formal definition, energy uses heat to establish equivalencies between vastly different scales of activity, from the formation of stars and the rusting of metals to the “vital force” of living creatures. The power of those comparisons can obscure other principles, such as the quality of different energy sources, their relative utility, and the conversion of energy into products, services, and forms of order with little measureable energy content. Each of these concepts helps illuminate critical aspects of environmental building design for which energy use or conversion efficiency are insufficient. To sort them out, we need to start at the beginning.
Thermodynamics really began in the shop, or rather at the mine head, when Thomas Savery, Thomas Newcomen, and James Watt developed steam engines to pump water from coal mines in the seventeenth and eighteenth centuries. Miners needed the engines because they had exhausted the easier-to-reach surface coal, driving miners below groundwater levels in search of coal (Galloway 1882). Of course, digging more aggressively for coal had only become necessary after loggers had depleted the forests of wood, which had helped fuel the growth in populations and industries that were hungry for other sources of power. In other words, steam made by burning coal was the latest step in which technical capacities and sources of available energy were developing together.
The foundation of thermodynamics was not the so-called “first” or universal law of energy conservation. Rather, it was the “second” law that developed first and explained the maximum efficiency with which those engines could convert the heat from burning coal into the work of pumping water. Sadi Carnot, in Reflections on the Motive Power of Fire (1824), first worked out the mathematical formulation of that efficiency, and Rudolf Clausius and Hermann von Helmholtz later formalized the definition of entropy that explained the second law of thermodynamics.
Twenty years later, in the 1840s, James P. Joule, Julius von Mayer, and Helmholtz consolidated a great deal within the sciences when they formalized the equivalency between heat and work in the “first” law of energy conservation. Ultimately, however, it is the second law that is actually useful. As the ecologist Robert E. Ulanowicz argues, “In ecology, as in all other disciplines that treat dissipative systems, the first law is not violated, but it simply does not tell us very much that is interesting about how a system is behaving” (Ulanowicz 1997, 24). The important question for the shop, the tidal estuary, or the human economy is how much energy is available to do useful work and how does it create systems of such intricacy? The interesting thing about the second law is that it depends on the conditions surrounding the work we hope to accomplish. In terms worked out by Carnot, the efficiency of a heat engine is limited by the temperatures in its immediate surroundings (the greater the temperature difference, the higher the efficiency). The second law also implies a broader account of the capacities for work that are available in the coal that is burned, the iron of the engine, or even in the social arrangements that made it possible to put the two together. Each of those examples, a concentrated fuel, a purified metal, and a working economic system, requires a considerable prior investment of work to make it available and represent a substantial amount of energetic potential.
Clausius’ simplest statement of the second law, that “heat cannot of itself flow from a colder body to a warmer one,” may not seem to capture all those different potentials (Clausius and Hirst 1867). Its fuller implications emerge from the concept of entropy, which is a quantification of the capacity that is lost when heat flows from a hotter to a colder body. Any transformation of energetic potential, whether into useful work or simply into lower temperature heat, irreversibly increases entropy. You cannot turn ashes back into coal or rust into iron without doing even more work to reassemble all the capacity that was released in the burning or rusting. The term “entropy” is somewhat counterintuitive: it describes the amount of energy that is unavailable to do work; it is often used synonymously with disorder or uncertainty; and it leads to the use of opposite terms such as negentropy, order, and information to describe the capacity for work that is built up throughout the environment. What we are interested in, however, is the capacity available to do work, whether of wood to heat a house or money to buy food.
Limitations of efficiency: the roles of entropy and exergy
Thermodynamics has largely been implemented with the measure of efficiency, and it has been one of the fundamental tools of technological innovation. Reviewing the different uses of efficiency helps reveal the difference between the different principles of thermodynamics. Count Rumford observed the heat equivalence of work in 1798, based on his measurements of the frictional heat released while boring cannons. The theory that forms the basis of the first law was not accepted until the 1840s, when Joule and Mayer independently showed the same correspondence (with more precision) in the interactions between mechanical work, electricity, magnetism, chemical reactions (combustion and digestion), and heat. The energy defined in the first law is ultimately the abstract quantity that is conserved in all those interactions, which is measured as heat. Efficiency measurements of the first law are therefore expressed in heat equivalents, comparing, for example, the heat content of the fuel burned in a machine to the heat value of the work delivered at the end.
What first-law equivalencies miss is the amount of work originally available in that fuel, which is the role of entropy. Entropy forms the basis of the second law of thermodynamics, whose definition was completed in the 1870s by J. Willard Gibbs when he added chemical potential to the description of a thermodynamic state. This enabled Gibbs to quantify the total free or available energy, which is now more commonly called exergy, and it better describes what energy means in common language (Rant 1956). According to the second law, exergy is destroyed in any process of energy conversion (while entropy increases). The second-law (or exergy) efficiency is the ratio of the work output to the exergy input. It has proved to be a more useful tool for the engineering of chemical processes and mechanical energy conversions, since it describes how much of the available energy has actually been exploited.
For building performance, exergy analysis is typically applied to explicit energy conversions, such as the burning of fuels or the use of electricity in mechanical and electrical equipment. The first-law efficiency of a natural gas furnace, for example, has increased dramatically in recent decades, from delivering 65% to 80% of the fuel’s heat content to ratings of around 97% today. But this only highlights the inadequacy of first-law measurements. A second-law efficiency would compare that delivery to the performance of an ideal heat pump working at its Carnot-defined coefficient of performance (COP) of about seven, meaning that it provides seven units of heat for each unit of electric power it consumes. This means that the most efficient natural gas furnace is only providing about 14% of the heat that could be delivered by an ideal heat pump.
Energy quality and the scope of analysis
Calculating second-law efficiencies highlights the importance of the boundaries of analysis that are used. For instance, the evaluation of the natural gas furnace could be put in larger context by including the fuels that were burned to produce the electricity to drive the heat pump, or even the work and resources required to build and operate the furnace. To understand the full environmental cost of a product or service, the boundaries of analysis have to be expanded to include the full array of inputs and effects required to provide the heat. The biosphere establishes ...