![]()
Part I
COGNITION AND NEUROSTRUCTURE
![]()
1
Specification of Higher Cortical Functions
Patricia S. Goldman-Rakic
Yale School of Medicine
One of the dramatic aspects of the behavioral profiles in autism, Turner, and Williams syndromes is that glaring deficits in one cognitive domain coexist with remarkable performance in another. The dissociation of capacity can be striking, as for instance in the capacity of Williams syndrome children to recognize faces and objects in noncanonical views although failing utterly to copy the simplest pictures of ordinary items (Bellugi, Klima, & Sipple, 1989). Understanding of how cerebral cortex is organized both anatomically and functionally can aid in interpreting the apparent contradictions on behavioral capacity and perhaps shed light on the etiology of disorders by identifying the structures, systems, circuits, and perhaps cell classes involved in a particular condition. Such information would lead to theories of why a particular subset of structures is more vulnerable than others to a genetic or epigenetic event.
A major issue in neurobiology of particular relevance to these developmental disorders is the degree to which specific functions are strictly localized in the central nervous system. The answer to this question has varied widely over this century. Earlier in this century, Lashley (1929) was impressed with the lack of specificity of cortical mechanisms because relatively large lesions rarely resulted in lasting behavioral deficits. Although few neuroscientists today would deny localization of function, many still view the cortex as capable of considerable restructuring as a basis for sparing of function after cortical damage. Another school of thought adheres to the more traditional view that the brain is essentially hard-wired and its plasticity limited. Whatever view is held, it is clear that the true degree of neural and behavioral plasticity requires a thoroughgoing analysis of function allocation in the normal brain and the detailed mechanisms underlying the latitude of these functions. Yet our understanding of normative structure-function relationships often falls far short of this prerequisite, particularly in the cerebral cortex.
Understanding of structure-function relationships is most advanced in the sensory cortices, particularly the primary visual and somatosensory areas. However, even with our knowledge of the anatomical and physiological organization of sensory cortices (Edelman, Gall, & Cowan, 1984), there is little consensus on the degree of their reorganization in the face of lesions and, in my opinion, only suggestive evidence that lost sensory functions can be reconstituted after damage to specified areas. Indeed, one of the currently more dramatic examples of cortical plasticity is the expansion of the face representation in the somatosensory cortex following deafferentation by dorsal rhizotomy in the rhesus monkey (Pons et al., 1991). This example demonstrates that intact functions can be enlarged but it does not show that lost functions can be recovered; in this case, an enlarged facial representation is at the expense of a diminished hand representation. Finally, in this widely publicized example of plasticity, it is by no means clear that the cortex rather than the lower centers has been reorganized.
It is of considerable importance that issues of functional and structural organization be examined in a variety of cortical areas. It is often tacitly assumed that the association areas would be those areas of the cortex most advanced with respect to plastic mechanisms. These uncharted territories have long been considered to be less specific in their organization and more adaptive to environmental contingencies. This chapter is about one area of prefrontal cortex—the principal sulcus—that has been the subject of intense study over several decades (Fuster, 1989; Goldman-Rakic, 1987). Our studies provide an opportunity to examine functional localization. The example from our work concerns critical aspects of spatial cognition, a complex cognitive domain that can be studied very well in monkeys and a domain that I believe demonstrates that higher cortical functions depend on specific processing mechanisms that are organized in a manner analogous to the more rigidly specified sensorimotor functions of the brain. Additionally, these studies reveal a remarkably strong relationship between the behavioral, anatomical, and physiological organization of a brain region and its function.
AREAL LOCALIZATION
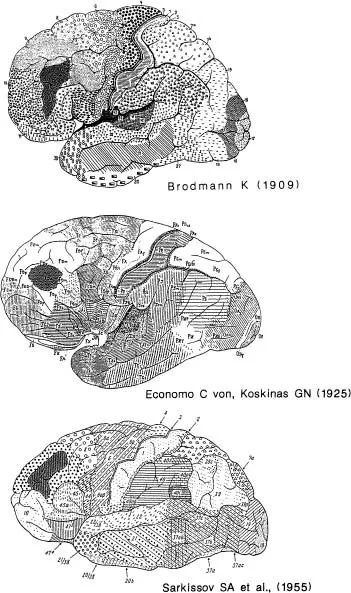
To understand the contribution of the cerebral cortex to behavior and higher cortical function, we have used the strategy of focusing on a particular area referred to in Brodmann’s and others’ cytoarchitectonic maps as Area 46 (Fig. 1.1). This area is adjacent to Area 9 and is one of many subdivisions in the prefrontal cortex. It is our conviction that if this area could be better understood in terms of anatomical circuitry, neurotransmitters, and their receptors and essential functions, we should be able to explain other prefrontal areas including Areas 44, 45, 11, 10, 9, 8, and all of the different subdivisions in the human prefrontal cortex that are thought to subserve the distinctively human capacities of reasoning, planning, and conceptual ability.
FIG. 1.1. Lateral views of classical cytoarchitectonic maps of human cerebral cortex with Area 46 shaded on each. Top: Brodmann’s map; Middle: von Economo and Koskinas (1925); Lower: Sarkissov, Filimonoff, & Kononova, (1955). Note that the shape and size of Area 46 is somewhat different in each map.
Figure 1.1 shows three classical maps of the human frontal lobe, one of Brodmann, one of von Economo’s, and one of Sarkissov’s. Examination of these maps reveals that Area 46 is slightly different in each of the maps. However, recently in my laboratory we reconstructed this area in a number of human brains on the basis of very specific cytometric criteria (Rajkowska & Goldman-Rakic, in press). To our surprise, we found that each of the first three brain reconstructions (not shown) conformed nicely to each of the three cytoarchitectonic maps, leading us to conclude that the classical anatomists were really quite accurate in their maps; they were just looking at different brains. Our findings thus emphasize that there are individual differences among humans in the size and shape of Area 46. On the other hand, we can emphasize the similarities in all of the brains we have examined, as Area 46 is always in the dorsolateral sector of the frontal lobe, occupying the middle frontal gyrus to one degree or other, and it is nearly always distinguishable from surrounding or neighboring regions by the distinctness of its inner granular IVth layer. Thus this area exists in roughly the same location in every normal human.
WORKING MEMORY
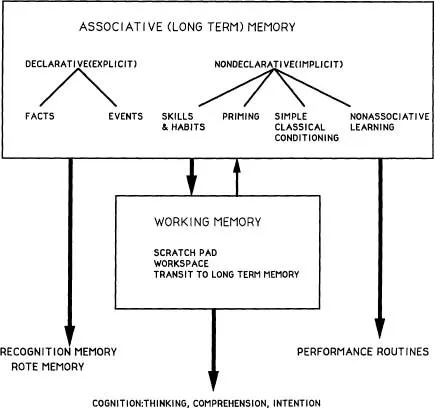
A vast clinical and experimental literature supports the conclusion that Area 46 and other areas in the prefrontal cortex are highly specialized for a particular kind of memory function (Goldman-Rakic, 1987). Although that function has been given different names, I favor the descriptive term working memory to denote a memory process defined by temporary coding and dynamic processing of memoranda in the workspace of the mind (see Baddeley, 1983; Carpenter & Just, 1988). Simply stated, working memory is the ability to hold information in mind, to internalize information, and use that information to guide behavior without the aid of or in the absence of reliable external cues. Working memory is a central concept in the study of language, comprehension, and reasoning (Baddeley, 1983; Carpenter & Just, 1988). An important point is that working memory is a very different process than associative memory, which is the process of acquiring knowledge by repetition and by reinforcement (see Goldman-Rakic, 1987, for further discussion of this point). Working memory to a large extent presupposes knowledge; it is a dynamic process that operates on the products of associative learning (Fig. 1.2).
FIG. 1.2. Diagrammatic representation of the relationship between Associative Memory and Working Memory Systems. Declarative and Nondeclarative Memory as partitioned by Squire (1991) is treated as one domain of behavioral control defined by dependence on the repetition of constant relationships over time. In this scheme, facts and events are remembered through memorial or actual repetition, as are habits. The contents of associative memory stores is the source of the material upon which working memory operates. Associative memory is the grist and working memory the mill. Information that is directly retrieved from associative memory stores is expressed as recognition and/or routine performance. However, if that information is processed dynamically, it can lead to comprehension, thought, and intentionality.
Can working memory be studied in animals? I have posited that the process referred to as working memory in humans is measured by classical delayed-response tasks given to experimental primates. In these tasks, a monkey watches an experimenter place a food morsel into one of two wells and a delay is imposed by lowering a screen and preventing the animal from orienting to the food well. After a delay, which can be as brief as a few seconds, the screen is raised. The animal has to select which of the two food wells contains the reward.
The important point that illustrates the nature of the distinction between working and associative memory is that at the time the animal must make its response, there is no information in the environment to guide the response. There is no cue, no signal, no differential discriminative stimulus in the situation external to the animal. The information that guides the response is in the animal’s “mind.” To achieve correct performance, it has to base its response on an internalized representation of a stimulus presented seconds before. The interposition of the delay between the input and the output forces the animal to use internalized information to guide its response.
LESIONS OF AREA 46
Bilateral removal of Area 46 produces a profound impairment on delayed-response tasks, particularly spatial delayed-response tasks, which require the animal to remember where an object is placed (for review, see Goldman-Rakic, 1987). It is important to recognize that the impairment is restricted to this task and similar tasks that require the animal to base its response on remembered spatial cues. Thus, monkeys with lesions of the principal sulcus are not at all impaired on cued spatial responses, for example (Goldman & Rosvold, 1970; Passingham, 1975). Furthermore, monkeys with dorsolateral prefrontal lesions are not impaired on any number of other tasks, in which the stimuli are external to the animals, are nonspatial, or require any association between stimuli and responses that is repeatedly reinforced (Goldman-Rakic, 1987). Similarly, patients with frontal lobe lesions can perform a wide range of associatively learned skills and may even achieve normal scores on IQ tests, reflecting that the store of associatively acquired knowledge is relatively intact (Hebb, 1949). In spite of an intact long-term memory system, the frontal lobe patient is deficient in using his knowledge to guide behavior in a coherent, goal-directed manner. Thus, the frontally damaged monkey and human provide strong examples of a remarkable dissociation between associative memory that is relatively intact and working memory that is deficient.
OCULOMOTOR DELAYED RESPONSE
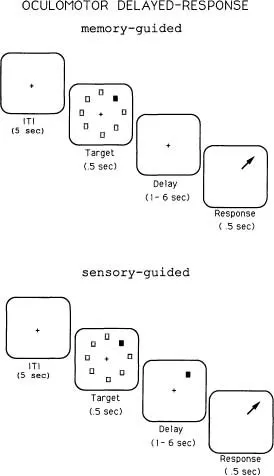
The classical delayed response has been used for decades to test spatial memory, and it has been instrumental in elucidating basic principals of brain and behavior. However, modern versions of this task have provided the opportunity for a more detailed analysis of brain-behavior relationships. In our laboratory, we employ an oculomotor version of the classical delayed-response task, which requires the monkey to fixate a small spot on the center of a cathode ray tube throughout a trial (Funahashi, Bruce, & Goldman-Rakic, 1989). As shown in Fig. 1.3, a trial consists of presenting a target in one of eight locations. The target is presented briefly, for half a second, and then goes off. As in the classical spatial delayed-response task, a delay is introduced. At the end of the delay, the fixation spot goes off, instructing the animal to direct its gaze to where the target had been. Important to note, as in the manual version, no information to guide the response is available at the end of the delay and the animal must base its response on an internalized cue. This task allows us not only to test the animal’s ability to keep in mind a previous event, but to differentiate memoranda of the exact X-Y coordinates of that event, that is, whether it was located at 90°, 45°, 135°, and so forth. In a fundamental way, the oculomotor delayed-response (ODR) paradigm allows manipulation of the contents of the animal’s memory on every trial. Further, given that the position of the target to be remembered is randomly varied from trial to trial, the test requires the animal to continuously update this information on a moment-to-moment basis. Finally, notice that if the target is left on during the delay period and is present at the end of the delay, we can compare the same responses when they are sensory guided as opposed to memory guided (lower panel, Fig. 1.3).
FIG. 1.3. This figure diagrams the oculomotor delayed-response task in its memorial version (top) and sensory-guided (bottom) versions. In the memory-guided version, the stimulus to be remembered (nght, upper quadrant) disappears before the delay and the animal makes a response in the absence of an external cue when the fixation spot extinguishes at the end of the delay. In the sensory-guided task, the cue remains on during the delay and is present to guide the response at the end of the delay.
MNEMONIC SCOTOMATA
In a recent study in our laboratory, rhesus monkeys received unilateral lesions around the principal sulcus or the adjacent frontal eyefields after they had learned to perform the ODR task at high levels (90% correct or better). All monkeys were impaired in making saccadic eye movements to remembered cues presented in the visual field contralateral to the hemisphere in which the lesion was placed. When a second lesion was added in the opposite hemisphere, the deficit was enlarged to include the remaining hemifield. The degree of impairment was related to the length of the delay; that is, it was exacerbated as the delay increased and errors consisted of eye movements in inappropriate directions. Significantly, the prefrontal lesions had no effect on eye movements that were sensory guided in the control task, even though those eye movements were of identical polarity to those that were impaired in the memory-guided version of the task. It is remarkable that lesions in the prefrontal cortex produce deficits only on the memory-guided version and not the sensory-guided version of the ODR task, proving once again that the functions...