![]()
chapter one
The element carbon
Frank Hennrich
Institut für Nanotechnologie
Candace Chan
Stanford University
Valerie Moore
Rice University
Marco Rolandi
Stanford University
Mike O’Connel
Theranos, Inc.
Contents
1.1 | Allotropes of carbon |
1.2 | History |
1.3 | Structure |
1.4 | Progress of single-walled carbon nanotube research |
References |
Carbon is the most versatile element in the periodic table, owing to the type, strength, and number of bonds it can form with many different elements. The diversity of bonds and their corresponding geometries enable the existence of structural isomers, geometric isomers, and enantiomers. These are found in large, complex, and diverse structures and allow for an endless variety of organic molecules.
The properties of carbon are a direct consequence of the arrangement of electrons around the nucleus of the atom. There are six electrons in a carbon atom, shared evenly between the 1s, 2s, and 2p orbitals. Since the 2p atomic orbitals can hold up to six electrons, carbon can make up to four bonds; however, the valence electrons, involved in chemical bonding, occupy both the 2s and 2p orbitals.
Covalent bonds are formed by promotion of the 2s electrons to one or more 2p orbitals; the resulting hybridized orbitals are the sum of the original orbitals. Depending on how many p orbitals are involved, this can happen in three different ways. In the first type of hybridization, the 2s orbital pairs with one of the 2p orbitals, forming two hybridized sp1 orbitals in a linear geometry, separated by an angle of 180°. The second type of hybridization involves the 2s orbital hybridizing with two 2p orbitals; as a result, three sp2 orbitals are formed. These are on the same plane separated by an angle of 120°. In the third hybridization, one 2s orbital hybridizes with the three 2p orbitals, yielding four sp3 orbitals separated by an angle of 109.5°. Sp3 hybridization yields the characteristic tetrahedral arrangements of the bonds. In all three cases, the energy required to hybridize the atomic orbitals is given by the free energy of forming chemical bonds with other atoms.
Carbon can bind in a sigma (σ) bond and a pi (π) bond while forming a molecule; the final molecular structure depends on the level of hybridization of the carbon orbitals. An sp1 hybridized carbon atom can make two σbonds and two πbonds, sp2 hybridized carbon forms three σ bonds and one πbond, and an sp3 hybridized carbon atom forms four σ bonds. The number and nature of the bonds determine the geometry and properties of carbon allotropes.
1.1 Allotropes of carbon
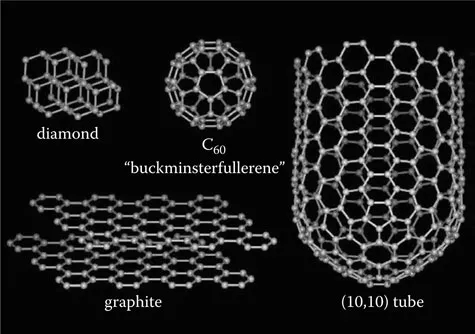
Carbon in the solid phase can exist in three allotropic forms: graphite, diamond, and buckminsterfullerene (Figure 1.1). Diamond has a crystalline structure where each sp3 hybridized carbon atom is bonded to four others in a tetrahedral arrangement. The crystalline network gives diamond its hardness (it is the hardest substance known) and excellent heat conduction properties (about five times better than copper).1 The sp3 hybridized bonds account for its electrically insulating property and optical transparency. Graphite is made by layered planar sheets of sp2 hybridized carbon atoms bonded together in a hexagonal network. The different geometry of the chemical bonds makes graphite soft, slippery, opaque, and electrically conductive. In contrast to diamond, each carbon atom in a graphite sheet is bonded to only three other atoms; electrons can move freely from an unhybridized p orbital to another, forming an endless delocalized πbond network that gives rise to the electrical conductivity.
Figure 1.1 The three allotropes of carbon. (From http://smalley.rice.edu/smalley.cfm?doc_id=4866.)
Buckminsterfullerenes, or fullerenes, are the third allotrope of carbon and consist of a family of spheroidal or cylindrical molecules with all the carbon atoms sp2 hybridized. The tubular form of the fullerenes, nanotubes, will be the subject of this book, and a detailed description of their history, properties, and challenges will be given in the next section.
1.2 History
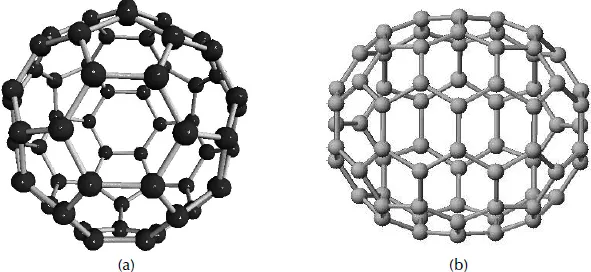
Fullerenes were discovered in 1985 by Rick Smalley and coworkers.2 C60 was the first fullerene to be discovered. C60, or “bucky ball,” is a soccer ball (icosahedral)-shaped molecule with 60 carbon atoms bonded together in pentagons and hexagons. The carbon atoms are sp2 hybridized, but in contrast to graphite, they are not arranged on a plane. The geometry of C60 strains the bonds of the sp2 hybridized carbon atoms, creating new properties for C60. Graphite is a semimetal, whereas C60 is a semiconductor.
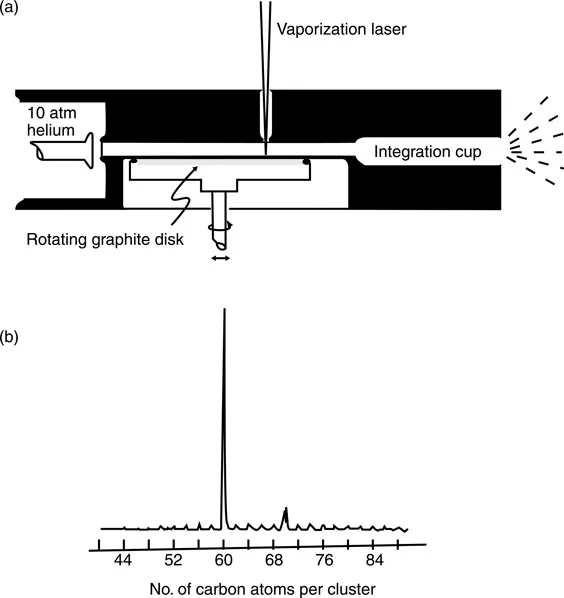
The discovery of C60 was, like many other scientific breakthroughs, an accident. It started because Kroto was interested in interstellar dust, the long-chain polyynes formed by red giant stars. Smalley and Curl developed a technique to analyze atom clusters produced by laser vaporization with time-of-flight mass spectrometry, which caught Kroto’s attention. When they used a graphite target, they could produce and analyze the long chain polyynes (Figure 1.2a). In September of 1985, the collaborators experimented with the carbon plasma, confirming the formation of polyynes. They observed two mysterious peaks at mass 720 and, to a lesser extent, 840, corresponding to 60 and 70 carbon atoms, respectively (Figure 1.2b). Further reactivity experiments determined a most likely spherical structure, leading to the conclusion that C60 is made of 12 pentagons and 20 hexagons arranged to form a truncated icosahedron2,3 (Figure 1.3).
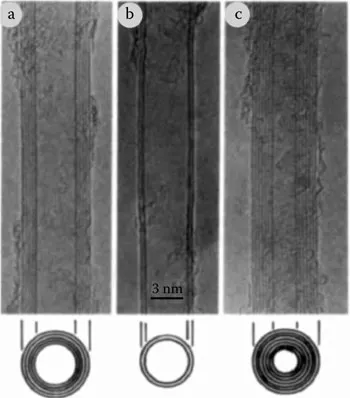
In 1990, at a carbon–carbon composites workshop, Rick Smalley proposed the existence of a tubular fullerene.4 He envisioned a bucky tube that could be made by elongating a C60 molecule. In August of 1991, Dresselhaus followed up in an oral presentation in Philadelphia at a fullerene workshop on the symmetry proposed for carbon nanotubes capped at either end by fullerene hemispheres.5 Experimental evidence of the existence of carbon nanotubes came in 1991 when Iijima imaged multiwalled carbon nanotubes (MWNTs) using a transmission electron microscope6 (Figure 1.4). Two years after his first observation of MWNTs, Iijima and coworkers7 and Bethune and coworkers8 simultaneously and independently observed single walled carbon nanotubes (SWNTs).
Figure 1.2 (a) Schematic of the pulsed supersonic nozzle used to generate carbon cluster beams. (b) Time-of-flight mass spectra of carbon clusters prepared by laser vaporization of graphite. (From H.W. Kroto, J.R. Heath, S.C. Obrien, R.F. Curl, and R.E. Smalley. C-60-Buckminsterfullerene, Nature, 318, 162–163, 1985.)
Although Ijima is credited with their official discovery, carbon nanotubes were probably already observed thirty years earlier from Bacon at Union Carbide in Parma, OH. Bacon began carbon arc research in 1956 to investigate the properties of carbon fibers. He was studying the melting of graphite under high temperatures and pressures and probably found carbon nanotubes in his samples. In his paper, published in 1960, he presented the observation of carbon nanowhiskers under SEM investigation of his material9 and he proposed a scroll like-structure. Nanotubes were also produced and imaged directly by Endo in the 1970’s via high resolution transmission electron microscopy (HRTEM) when he explored the production of carbon fibers by pyrolysis of benzene and ferrocene at 1000°C.10 He observed carbon fibers with a hollow core and a catalytic particle at the end. He later discovered that the particle was iron oxide from sand paper. Iron oxide is now well-known as a catalyst in the modern production of carbon nanotubes.
Figure 1.3 Models of the first fullerenes discovered, C60 and C70.
Figure 1.4 Transmission electron micrographs (TEMs) of the first observed multi-walled carbon nanotubes (MWNTs) reported by Iijima in 1991. (From S. Iijima. Helical microtubules of graphitic carbon, Nature, 354, 56–58, 1991.)
Although carbon nanotubes were observed four decades ago, it was not until the discovery of C60 and theoretical studies of possible other fullerene structures that the scientific community realized their importance. Since this pioneering work, carbon nanotube research has developed into a leading area in nanotechnology expanding at an extremely fast pace. Only 9 papers containing the words “carbon nanotube” were published in 1992 and over 5000 publications were printed in 2004. All this interest in this new form of material was triggered by its unique properties and numerous potential applications, which will be described in the next sections.
1.3 Structure
Iijima was first to recognize that nanotubes were concentrically rolled graphene sheets with a large number of potential helicities and chiralities rather than a graphene sheet rolled up like a scroll as originally proposed by Bacon. Iijima initially observed only MWNTs with between 2 and 20 layers, but in a subsequent publication in 1993, he confirmed the existence of SWNTs single-walled carbon nanotubes and elucidated their structure.7
A SWNT is a rolled graphene sheet. Although the growth mechanism does not suggest a carbon nanotube is actually formed like a sushi roll, the way the graphene sheet is rolled determines the fundamental properties of the tube.
In order to describe such a fundamental characteristic of the nanotube, two vectors, Ch and T, whose rectangle defines...