![]()
Chapter 1
Recent Trends in Sol-Gel-Based Nanoceramics
Pradeep Pratap Singha and Ambikab
a Department of Chemistry, Swami Shraddhanand College, University of Delhi, New Delhi 110036, Delhi, India
b Department of Chemistry, Hans Raj College, University of Delhi, New Delhi 110007, Delhi, India
Nanoceramics refer to ceramic materials fabricated from ultrafine particles. They have attracted the interest of researchers and scientists due to potential to achieve better and some unusual material properties by manipulating length scale in the nanorange. The synthesis of nanocrystalline powders is an essential step in the processing of nanoceramics. Various methods have been reported to synthesize nanocrystalline powders to ensure appropriate control of particle size, surface contamination, and degree of agglomeration. Recently the sol-gel route has been utilized for the synthesis of nanoceramics. The sol-gel methods have the ability to produce a large variety of compositions and ensure homogeneous mixing of the constituent particles at low temperature. This method can be used in ceramic processing, in manufacturing, and for producing very thin films of metal oxides for various purposes. Sol-gel-derived nanoceramics have numerous applications in different technological areas. This chapter deals with the recent trends in sol-gel-based nanoceramics and their applications.
1.1 Introduction
The field of ceramics has utilized nanoscience and nano-technology for producing a variety of advanced materials with unique properties and performance. Ceramic nanocomposites are attracting growing interest due to their wide range of applications. “Nanoceramics” is a term used to refer to ceramic materials fabricated from particles less than 100 nm in diameter. They encompass many forms of ceramics where at least one of the dimensions is in the nanoscale range (Biest, 2013). Nanoceramics exhibit unique mechanical and surface characteristics, such as superplasticity, machinability, strength, toughness, and bioactivity due to the fine grain size, abundant grain boundaries, and controllable crystallinity (Kiani et al., 2014). However, their processing is the major challenge in research. The basic steps in nanoceramic fabrication involve synthesis of unagglomerated nanosized powders with uniform size distribution and sintering without grain growth. Various chemical methods have been adopted to synthesize nanocrystalline powders to ensure appropriate control of particle size, surface contamination, and degree of agglomeration. Recently, the sol-gel method has been utilized for the synthesis of nanoceramics (Perera, 2010; Owens et al., 2016).
Sol-gel processing is a liquid-phase processing of nanocomposite powders that utilize inorganic salts or metal-organic compounds for sol preparation. The sol is converted into a gel by hydrolysis and condensation reactions, which is then dried to eliminate the excess liquid phase. The gel shrinks and transforms to the desired phase after drying, leading to complex shapes, directly from the gel state. This process controls the homogeneity of chemical composition and lowers the processing temperatures. In addition, by controlling the gelation parameters and subsequent thermal treatments, it is possible to tailor the microstructure. Even small quantities of dopants, such as organic dyes and rare earth elements, can be introduced in the sol and end up uniformly dispersed in the final product (Hafez, 2012). Sol-gel processing can be used in ceramic processing, in manufacturing, and for producing very thin films of metal oxides for various purposes (Palmero, 2015). Sol-gel-derived nanoceramics have diverse applications in optics, electronics, energy, space, sensors, medicine, corrosion protective coatings, engineering, and biomaterials science (Zhao et al., 2008; Simchi et al., 2011; Uche, 2013; Senthil et al., 2014).
1.2 Classification of Ceramic Nanocomposites
Ceramic nanocomposites can be broadly classified into the following categories (Basu et al., 2006):
• Intergranular nanocomposites: These nanocomposites possess nanosized reinforcements at the grain boundaries and triple junctions of the matrix grains, which pins in the grain boundaries from migration, which results in improved creep resistance.
• Intragranular nanocomposites: These consist of nanoscaled reinforcements within the matrix grains, which results in high strength and toughness at room temperature.
• Inter-/intragranular nanocomposites: These nanocomposites show a combination of properties of the first two classes. They have nanosized reinforcements at the grain boundaries, at the triple junctions, and within the matrix grains. The hardness, toughness, strength, fracture resistance for creep, and fatigue at high temperatures, as well as the thermal shock fracture resistance, are strongly improved for these composites (Niihara et al., 1999).
• Nano-/nanocomposites: In these nanocomposites both the matrix and the reinforcement particulates are in the nanosized range. They exhibit superplasticity and superior machinability due to enhanced grain boundary sliding.
• Nano-/microcomposites: In this type micron-size particulates are dispersed in a nanosized matrix (Basu et al., 2006).
1.3 Sol-Gel Methods and Chemistry
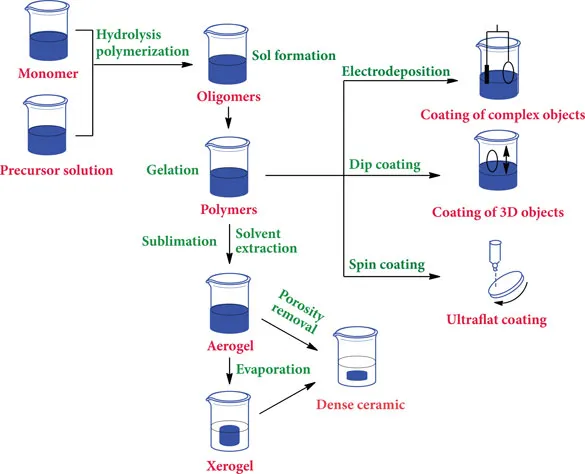
Sol-gel processing is one of the recent synthesis routes that are used to produce nanoceramics. All sol-gel methods involve two distinct phases, solution and gelation. A sol is a colloidal suspension of solid particles, whereas a gel is an interconnected network of solid-phase particles that form a continuous entity throughout a secondary phase that is generally liquid. The presence of a network formed by the interlocking of particles of the gelling agent gives rise to the rigidity of a gel. The nature of the particles and the type of form that is responsible for the linkages determine the structure of the network and the property of the gel. Aerogels are sol-gel-derived solid materials with porosities of about 80%–98%. The high porosity is achieved through supercritical drying of wet gel in an autoclave (Roy, 1999; Ulrich, 2004). In sol-gel technology, these phases are conserved though the chemical reactions that take place during the gel evolutions and can be manipulated in a variety of ways, such as by altering the initial precursors, time for gelation, catalysts, degree of solvation, gelation conditions, or physical processing of the gel, depending on the specific application (Fig. 1.1).
Figure 1.1 Different steps of sol-gel synthesis and its applications.
There are four methods for the production of gels: (i) flocculation of lyophilic colloids by salts or precipitating liquids, (ii) evaporation of certain colloidal solutions, (iii) chemical reactions that lead to a change in the shape of lyophilic molecules (e.g., the denaturation of albumen on heating involves some uncoiling of the protein molecules and a gel structure results), and (iv) swelling of a dry colloid (xerogel) when placed in contact with a suitable liquid (e.g., starch granules added to water).
1.3.1 Colloidal Sol-Gel Methods
Colloidal solutions can be defined as solutions containing discrete particles that do not settle but remain suspended for several years (Iler, 1979). Colloidal sol-gel methods are an active area of research for producing uniform particle sizes (Hench et al., 1990). The applicability of the colloidal methods is based on two key aspects of the process: stabilization of the colloidal particles within the sol and flocculation to form the gel. Aggregation of colloidal particles can be achieved by the removal of the solvent, altering the pH, salinity, or temperature (Colomban, 1989). In most cases, colloidal sol-gel methods contribute to stabilizing the sensitive molecules. The advantage of colloidal methods over alkoxide-based systems is that the majority of the network is already present in the sol. Therefore, certain adaptations, such as the introduction of osmoprotectants, could be applied without significantly interfering with the integrity of the inorganic capsule itself (Perullini et al., 2011).
1.3.2 Polymer-Assisted Sol-Gel Methods
These methods involve the chelation of reactive inorganic gel-forming agents within an organic polymer network, depending on the material to be produced (Omori et al., 2014). The gel-forming agents are dispersed throughout the solution, thereby preventing the precipitation of aggregates within the sol (Kakihana, 1996). However, this method requires subsequent heat treatment to remove the organic polymer following the formation of the inorganic gel. This route offers an effective means of synthesizing materials that disperse poorly in viscous solutions or that would otherwise form reaction products prior to assembly into the required form. This is especially useful for the calcium phosphates (CaPs), which have a tendency to form a diverse range of minerals when present at low concentrations in aqueous solutions (Larsen, 1986). Recently nanoscale LiMn2O4 particles using Pluronic P-123 as a stabilizing polymer and citric acid as a chelation agent have been synthesized (Yang et al., 2013). The nanoparticle exhibited a superior degree of porosity when compared to particles that were synthesized without the presence of the polymer network. Chemical properties, such as the biomimetic molar ratios of apatites, can also be achieved with polymer-assisted stabilization due to the homogeneous elemental distribution of the gel network (Omori et al., 2014). To control the spatial distribution of calcium within a silica sol-gel network synthesized via a tetraethylorthosilicate (TEOS) precursor with 3-glycidoxy-propyltrimethoxysilane as the covalent coupling agent c-glutamic acid polymers have been utilized (Valliant et al., 2013). A homogeneous distribution of calcium in the silica network and the larger polymers also reduce the degradation rates. Inorganic networks can also be formed in situ through the polymerization of organic precursors. This method involved the formation of a 3D polyester network as a result of the reaction between ethylene glycol and citric acid (Pechini, 1967).
1.4 Applications of Sol-Gel in Nanoceramics
1.4.1 Nanosized Films and Nanostructured Coatings
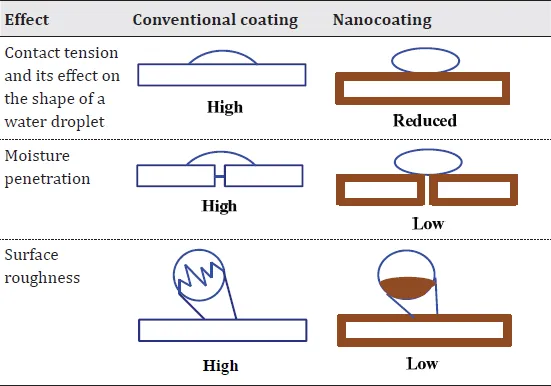
Sol-gel techniques have been utilized to change the functionalities by patterning the surface of a glass or another material that provides more degrees of freedom compared with uniform thin films or coating. In the traditional sol-gel method, hydrolysis-condensation processes are followed by condensation polymerization upon film application. Sol-gel preparations are cost effective, are simple to set up, and have the ability to coat complex shapes, similar to biomimetic coatings. The major advantage of sol-gel applications over biomimetic coatings is the strength of the coating-substrate adhesion (Qu et al., 2008; Zhang et al., 2006). However, the evaporation process results in voids and channels throughout the solid gel and cannot provide adequate corrosion protection. Also, the maximum coating thickness attainable by a sol-gel is lower than 2 mm, which can be increased by the incorporation of nanoparticles in the sol without increasing the sintering temperature (Castro et al., 2004). Incorporation of nanoparticles in the hybrid sol-gel systems increases the corrosion protection properties due to lower porosity and lower cracking potential (Table 1.1) (Zheludkevich et al., 2005). Incorporation of inorganic nanoparticles can insert corrosion inhibitors, preparing inhibitor nanoreservoirs for self-repairing pretreatments with controlled release properties (Zheludkevich et al., 2006). Sol-gel films containing zirconia nanoparticles have improved barrier properties. An additional improvement to corrosion protection is observed when cerium nitrate is doped to the above film. Zirconia particles present in the sol-gel matrix act as nanoreservoirs, providing prolonged release of the cerium ions (Zheludkevich et al., 2005).
Table 1.1 Effect of different types of coatings on the surface of the material
The zirconium-based conversion coatings (ZrCCs) on cold-rolled steel (CRS) substrates have the maximum corrosion resistance values and uniformity (Mohammadloo et al., 2014). An inorganically cross-linked (silica) sol-gel coating has been used to generate complex patterns (Letailleur et al., 2010). Recently, an aqueous sol-gel process of forming function...