
- 696 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
An Introduction to Quantum Physics
About this book
Provides comprehensive coverage of all the fundamentals of quantum physics. Full mathematical treatments are given. Uses examples from different areas of physics to demonstrate how theories work in practice. Text derived from lectures delivered at Massachusetts Institute of Technology.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Simple models of the atom
1-1 INTRODUCTION
We know that classical physics, as represented by Newtonian mechanics and Maxwell’s laws of electromagnetism, works marvelously well for the analysis of the behavior of macroscopic objects in terms of empirically determined laws of force. But as soon as we enter the world of the atom, we find that new phenomena appear, requiring new concepts for their analysis and description. The whole realm of phenomena at the atomic or subatomic level is the special province of quantum theory. However, because the behavior of matter in bulk ultimately results from the properties of its constituent atoms, our deeper insights into physical phenomena on the macroscopic scale will often also depend on quantum theory. For example: We can do a vast amount of useful analysis of the mechanical behavior of solids using measured values of their elastic constants, tensile strengths, etc. But if we want to account for these measured values in terms of more fundamental processes, we must invoke quantum theory. It is at the root of our whole understanding of the structure of matter.
The properties of atoms—and even the fact of their existence—pose a series of questions unanswerable by classical physics:
Atoms are typically a few angstroms in diameter (1Å = 10−8 cm) with remarkably little difference in size between the lightest and the heaviest (see Figure 1-1).

Fig. 1-1 Atomic radii. There is very little increase in atomic size with increasing atomic mass number. Note the periodic variation in radius, with the maximum radii being those of the alkali atoms.
Why this size rather than some other? And why not a wide range of sizes?
When isolated from radiation and other atoms, most atoms remain stable indefinitely: they neither collapse nor explode. Why do not the negatively charged electrons collapse into the positively charged nucleus, thereby destroying the atom to the accompaniment of a burst of radiation?
When atoms are excited electrically or by collisions or otherwise, they emit radiation of discrete wavelengths characteristic of the kind of atoms excited (see Figure 1-2). Why discrete wavelengths rather than a continuous spectrum? And how can a particular spectrum be accounted for, as well as differences between spectra of different kinds of atoms?
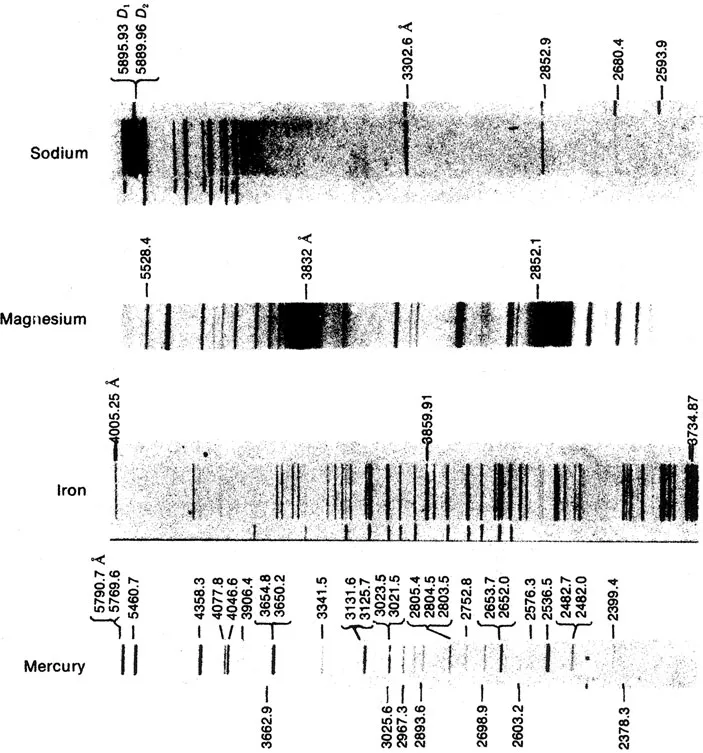
Fig. 1-2 Emission spectra of various vapors. The pattern of emission lines in each spectrum is characteristic of the particular chemical element. (Spectra reproduced from G. Herzberg, Atomic Spectra and Atomic Structure, Dover Publications, Inc., New York, 1944. Reprinted through the permission of the publisher.)
These questions are only a beginning. Why are some kinds of atoms more reactive chemically than other kinds? Why are some substances harder, denser, more transparent, more elastic, more electrically conductive, more thermally conductive, more digestible than other substances? All such questions can be related to the properties of atoms, and we can understand them only if we possess the facts and concepts embodied in quantum mechanics.
In this book we will turn our attention again and again to the atom, each time from a different point of view or level of sophistication. In the present chapter we discuss a few of the simplest models of the atom, all of them basically classical in nature, with one or two additional assumptions to help the classical models behave more like the observed quantum systems. Despite their crude nature these models can be used to correlate, even if they cannot be said to explain, a wide range of observations. The ultimate failures of these models force us to look more deeply and to return repeatedly to the atom with models of increasing sophistication.
Why start with crude models? Why return again and again to them when the “real” answers are already known?
Why not tell the quantum-mechanical truth straight out and then stop? Some readers may feel equipped to go straight to the now-accepted answers, and they can begin their study with a later chapter of this text. But for most people crude atomic models provide a gradual conceptual transition from classical descriptions to the “true” quantum statements about atoms, statements that seem strange and awkward at first but later on become comfortable, simple, and natural.
1-2 THE CLASSICAL ATOM1
The simplest model of the atom is a hard, tiny, electrically neutral sphere—just the smallest possible fragment of the bulk material that still possesses the identity of a given chemical element. According to this conceptually primitive picture, atoms (and molecules formed from them) exert no forces on one another until they are brought in contact, and then they offer infinite resistance to being forced any closer together. The dramatic difference in behavior between a substance in vapor form, on the one hand, and in its solid or liquid phase, on the other, is roughly consistent with such a model. This major difference in behavior does not involve a big change in interatomic or intermolec...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- PREFACE
- LEARNING AIDS FOR QUANTUM PHYSICS
- 1 Simple models of the atom
- 2 The wave properties of particles
- 3 Wave-particle duality and bound states
- 4 Solutions of Schrodinger’s equation in one dimension
- 5 Further applications of Schrodinger’s equation
- 6 Photons and quantum states
- 7 Quantum amplitudes and state vectors
- 8 The time dependence of quantum states
- 9 Particle scattering and barrier penetration
- 10 Angular momentum
- 11 Angular momentum of atomic systems
- 12 Quantum states of three-dimensional systems
- 13 Identical particles and atomic structure
- 14 Radiation by atoms
- ANSWERS TO EXERCISES
- BIBLIOGRAPHY
- SELECTED PHYSICAL CONSTANTS AND CONVERSION FACTORS
- INDEX
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access An Introduction to Quantum Physics by A.P. French in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Quantum Theory. We have over one million books available in our catalogue for you to explore.