
- 216 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Demonstrating Science with Soap Films
About this book
Many of us have been fascinated as children by soap bubbles and soap films. Their shapes and colours are beautiful and they are great fun to pay with. With no les intensity, scientists and mathematicians have been interested in the properties of bubbles and films throughout scientific history.
In this book David Lovett describes the properties of soap films and soap bubbles. He then uses their properties to illustrate and elucidate a wide range of physical principles and scientific phenomena in a way that unifies different concepts. The book will appeal not only to students and teachers at school and university but also to readers with a general scientific interest and to researchers studying soap films.
For the most part simple school mathematics is used. Sections containing more advanced mathematics have been placed in boxes or appendices and can be omitted by readers without the appropriate mathematical background.
The text is supported with
* Over 100 diagrams and photgraphs.
* Details of practical experiments that can be performed using simple household materials.
* Computer programs that draw some of the more complicated figures or animate sequences of soap film configurations.
* A bibliography for readers wishing to delve further into the subject.
David Lovett is a lecturer in physics at the University of Essex. His research interests include Langmiur-Blodgett thin films and the use of models as teaching aids in physics. He has been interested in soap films since 1978 and has made a number of original contributions to the subject, particularly in the use of models which change their dimensions and their analogy with phase transitions. He has published three other books including ITensor Properties of Crystals (Institute of Physics Publishing 1989).
John Tilley is also a lecturer in physics at the University of Essex with research interests in theoretical solid-state physics and soap films. He is coauthor of Superfluidity and Superc
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Introduction
The nature of soap films and films produced by other surfactants is described. The importance of surface energy is explained, and minimal-area surfaces are introduced. It is the comparison of minimization of energy and other quantities in physics with the minimization of area of a soap film which underlies all subsequent topics in this book.
1.1 WHAT ARE SOAP FILMS?
To create a soap film we must first produce a soap solution consisting of soap molecules and water molecules. Soap molecules consist of sodium stearate (or some other fatty acid salt), although the term soap film is now a generic term for a wide range of other molecules which produce similar effects. Sodium stearate is a long-chain fatty acid of composition C17H35COONa. Immersed in water, the molecules become ionized. The sodium ions have positive charge and disperse throughout the solution. This leaves a negatively charged head to the stearate ion which has a long hydrocarbon tail. There is a nett force on the stearate ions tending to take them to the surface to leave the hydrocarbon tails sticking out (figure 1.1(a)). Because these tails (C17H35) stick out of the water, they are often described as hydrophobic or water-hating and the so-called carboxyl head (COO-) as hydrophilic or water-loving. Some of the stearate ions remain within the bulk solution and play no part in the film. The same arguments apply if one uses artificial soaps and in particular the synthetic detergents as used for household washing- up liquids. Such molecules are often called surfactants because of the way they establish themselves at the surface.
Given sufficient encouragement via the processes of blowing bubbles or dipping frameworks into them, soap solutions can be induced to establish films in which two parallel surfaces of surfactant molecules are set up with water solution between (figure 1.1(b)). The surfactant ions have a length of approximately 3 nm (30 Å) and an area of 0.40 nm2 (40 Å2). The actual films can have a wide range of thicknesses varying from approximately 5 nm (50 Å) to ∽ 104 nm (105 Å) according to the volume of solution between the surfactants. We shall return to the topic of film thickness and the corresponding interference colours later in the book (see page 77).
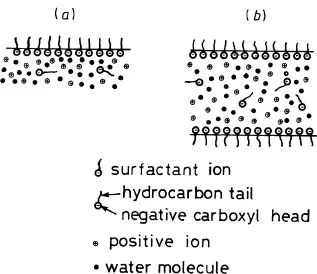
Figure 1.1 (a) Structure at the surface of a soap solution. (b) Structure through a soap film.
The lifetime of pure soap films is very sensitive to impurities such as dust particles and components such as excess fat. This does not apply in the case of modern detergents. Using tap-water plus 1 to 2 per cent washing-up liquid (liquid detergent), such as Sunlight (in the UK) or Dawn and Joy (in the USA)†, will produce films which last for at least 15 seconds. They can last considerably longer within some of the frameworks to be discussed later. Higher concentrations of washing-up liquid, say 10 per cent concentration, should enhance the lifetime of the films further. A moist atmosphere increases lifetime, whereas films tend to be short-lived when demonstrated above an overhead projector. Adding glycerol increases the lifetime of the films to times of minutes with say 5 per cent glycerol and to hours for 50 per cent glycerol. However, adding glycerol, if not adequately mixed in, can produce problems of cleaning the frameworks after use, whereas the lifetime achieved with commercial liquid detergent is usually adequate. It is very important that the detergent is thoroughly stirred into the water and does not form a residue on the bottom of the container. Leaving the solution for a time prior to use is helpful.
Recipes have been given for solutions which produce longer- lasting films and in particular Stong (1969) has described a number of alternative solutions which can be used. A solution first described by Plateau (1873) can be produced as follows. To 1200 g of distilled water add 30 g of sodium oleate. The latter which should be chemically pure is a white powder that floats on the surface of the water. Rather than mixing the powder in with the water, it is advised to allow the water plus powder to stand for 24 hours by which time the powder will have dissolved. At this stage, 30 g of glycerol is added and the solution mixed by pouring it back and forth between two containers. The mixture is stored in darkness for a week after which time the clear fluid which is formed below a scum on the top is syphoned off. A few drops of ammonia are added and the solution stored for use. Bubbles from this solution last for minutes.
A recipe reported by Jearl Walker (1987a) in Scientific American for producing rigid films is as follows:
100 g glycerine (85% solution)
1.4 g triethanolamine
2.0 g oleic acid
The chemicals are mixed and stored for 24 hours in the dark in an airtight bottle. If the solution is not clear at this stage, a little more triethanolamine should be added.
Kuehner (1958) described the preparation of solutions which enable bubbles to last for years. His method is very detailed and requires control of temperature often to below −20 °C. First oleic acid is purified. Secondly the purified oleic acid is converted to 9,10-dibromostearic acid by the addition of bromine. Thirdly, sodium 9,10-dibromostearate solution is produced by carefully neutralizing the acid with hydrogen dioxide and mixing- in glycerol. This solution produces long-lasting bubbles. This method of Kuehner, and a further method of Stong in which two solutions are mixed, one being Kuehner’s solution and the other being a solution of polyvinyl alcohol, water and glycerol, are not advised for the home experimenter. Rather the methods are mentioned to illustrate that for the experiments described in this book the use of selected commercial washing-up liquids is both adequate and very much easier.
1.2 SURFACE TENSION AND SURFACE ENERGY
Molecules near to the surface of a pure liquid do not exist in a uniform environment as do those within the interior of the liquid. They experience a weaker force in the direction of the gaseous region outside the liquid than in a direction towards the bulk liquid. Hence the molecules experience a nett force pulling them inwards and this has the effect of trying to reduce the volume of the liquid. This inward force is opposed by the outward forces between the molecules within the interior of the liquid as the molecular separation infinitesimally decreases. In addition, the resulting density of the liquid is slightly less at the surface. Thus we can see why water droplets take up a spherical shape. There is a good analogy with the situation of a balloon. The stretched rubber of the balloon attempts to pull the outer surface inwards and the pressure within the balloon counteracts this effect.
For the surface of the liquid to be in a state of uniform surface tension, the surface tension must be perpendicular to any line drawn in the surface. It must also have the same magnitude for all directions of the line within the surface, and it must have the same value at all points over the surface. In the case of a film, there are two parallel surfaces and hence an overall force of double magnitude.
The consequence of the above can be shown rather nicely using a wire hoop and a length of thread. The hoop is dipped into soap solution and removed to produce a uniform film. A loop of the thread is placed on the film and the film punctured within the loop. As a result the thread is pulled outwards by the film to form a very precise circle (figure 1.2). Now it is a property of a circle that it possesses maximum area for its given perimeter. More importantly, the film outside the thread takes on minimum area and as we shall soon realize this minimizes the energy associated with the film.
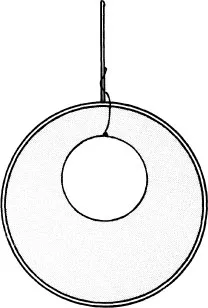
Figure 1.2 Demonstration of minimization of film area.
The above demonstration gives us the hint as to how we might define and measure the surface tension γ. We shall define γ as the force per unit length acting perpendicular to one side of a line within the surface of a liquid; but so that we can measure its value we shall need to have a film on one side of the line and air on the other. A suitable method of measuring the value is to use a sliding wire moving across a fixed frame (figure 1.3). We apply a force F to the right of the wire to balance the force due to surface tension on the left. At balance, the force arising from the surface tension will be 2γl, where l is the length of the sliding wire and the factor of 2 arises because there are two film surfaces. The surface tension of water is approximately 73 × 10−3 N m−1, but for soap solutions or oil or methylated spirit the values are considerably less. Hence, if one drops a little of any of these other liquids onto the surface of the water, assuming that the water surface is very large, the additive spreads out to form a monolayer.
The arrangement of figure 1.3 allows us to obtain an expression for the surface energy of the film. Suppose we do work using a force F (strictly we use a force infinitesimally greater that F) to move the wire from position x to position χ + δx. The work done will be 2γδx = 2γδA where δA is the change in area of the film. The total energy to produce a film of area A, starting from zero area, will be
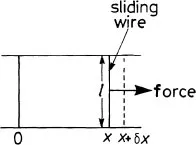
Figure 1.3 Framework to measure surface tension.
where E is the free energy of the film at constant temperature. The important feature is that the free energy of the film is proportional to its area as is demonstrated by the equation. Soap films attempt to minimize their energy and hence this means that they attempt to minimize their area. This minimization is fundamental to what follows in this book and underlies a large number of the demonstrations and analogues which can be shown using soap films.
1.3 A BRIEF HISTORY OF SOAP-FILM STUDIES
The understanding of the formation of various soap-film patterns is intimately linked with the mathematics of the calculus of variations and various principles of minimization. Many well- known mathematicians have used soap films to help them to understand or demonstrate their mathematical ideas. So perhaps we should start with Maupertuis (1698–1759) who stated his general principle that
‘if there occurs some change in nature, the amount of action necessary for this change must...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Frontispiece
- Preface
- 1 Introduction
- 2 Two-dimensional soap-film patterns
- 3 Soap films and first- and second-order phase transitions
- 4 Soap-film models and catastrophe models
- 5 Film within a wedge—the catenoidal surface
- 6 Soap films within three-dimensional frameworks and minimal surfaces
- 7 Fermat’s principle and refraction
- 8 Bubbles
- 9 Analogies within the scientific world
- Appendices
- Bibliography
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Demonstrating Science with Soap Films by Lovett in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physics. We have over one million books available in our catalogue for you to explore.