
eBook - ePub
Chemical Processes for Pollution Prevention and Control
This is a test
- 232 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Chemical Processes for Pollution Prevention and Control
Book details
Book preview
Table of contents
Citations
About This Book
This book examines how chemistry, chemical processes, and transformations are used for pollution prevention and control. Pollution prevention reduces or eliminates pollution at the source, whereas pollution control involves destroying, reducing, or managing pollutants that cannot be eliminated at the source. Applications of environmental chemistry are further illustrated by nearly 150 figures, numerous example calculations, and several case studies designed to develop analytical and problem solving skills. The book presents a variety of practical applications and is unique in its integration of pollution prevention and control, as well as air, water, and solid waste management.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Chemical Processes for Pollution Prevention and Control by Paul Mac Berthouex,Linfield C. Brown in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
1 The Chemical Process Design Problem
1.1 INTRODUCTION
Think of a modern city as a giant chemical process or a giant organism. It needs to be provided with food and energy, and it must rid itself of wastes. Figure 1.1 shows the transformation of water, food, and fuel into sewage, air pollutants, and solid wastes, and also into useful products. The inputs are broken down by various means and reorganized into different components, many of which are wastes. Coal becomes carbon dioxide, water, ash, sulfur dioxide, and other pollutants. Food becomes waterborne waste and refuse. Unwanted newspapers, cans of tomatoes, computers, and automobiles are discarded as refuse, which is often processed to recover reusable material.
Albert Einstein said that the environment was “everything that is not me.” For me to be healthy and happy, everything that is not me must be healthy and happy. The challenge is to maintain a healthy balance between the demands of 7 billion people and the natural cycles of essential nutrients and to include protection from hazardous substances.
1.2 CHEMICAL PROCESSES
Interesting and important chemical reactions occur in nature. Photochemical smog and acid rain are examples of chemistry in the atmosphere. Pollution problems in lakes, rivers, sediments, soil, and groundwater are caused by a failure to control the discharge of pollutants, either intentional or accidental. Contamination of soil and groundwater is caused by careless disposal and spills of chemicals.
The focus of pollution prevention and control is to minimize the flow of pollutants to the natural environment—the atmosphere, sediments, soil, streams, lakes, and oceans. This book is about using chemical processes to control pollutants before they can cause problems in the natural environment.
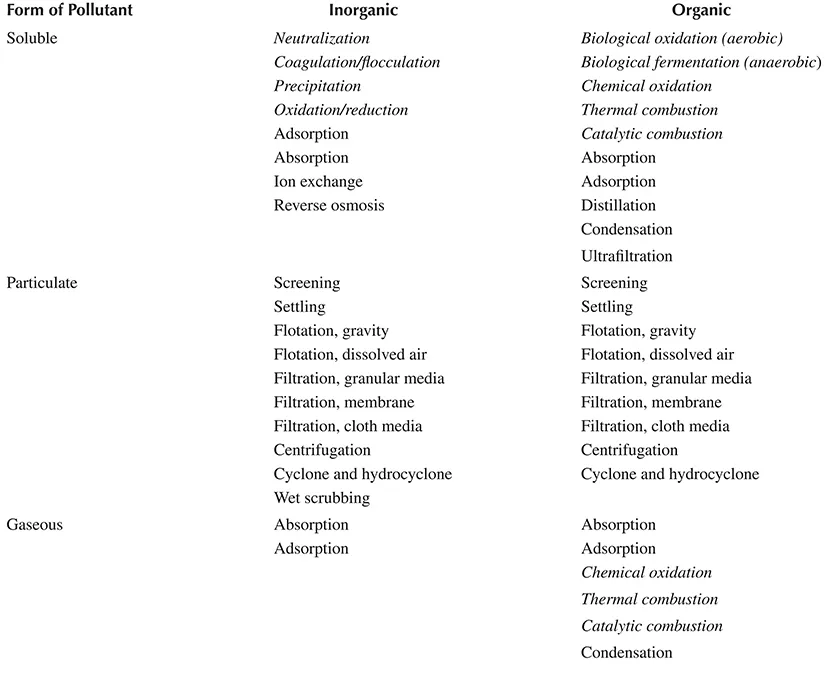
Table 1.1 classifies treatment processes according to whether they are best suited to handling inorganic or organic substances in soluble, particulate, or gaseous form. The italicized names are chemical processes. The rest are separations.
This book is mostly about soluble inorganic chemicals—the top left portion of Table 1.1. Selected topics about soluble and gaseous organic chemicals are also included.
Inorganic atoms and molecules can be rearranged, dissolved, and made to precipitate or agglomerate, and they can be diluted and concentrated. They are not biodegradable or combustible.
Organic compounds are biodegradable (at least most of them are). The biological reactions are oxidations and reductions, but usually we do not know specifically which chemicals are being reacted because wastewater is a mixture of many substances. Biological treatment processes must operate within fairly narrow limits of pH and temperature. These factors make them different than the most widely used inorganic chemical treatment processes. Biological transformations are not included in this book.
Organic chemicals vaporize and condense, and they adsorb and desorb. This suggests a class of treatment methods that are more separations than chemical reactions.
Air pollution control is mostly about physical separations, and to a lesser extent about chemical reactions and transformations. Some air pollution control processes capture the pollutants in a liquid stream. The absorption of sulfur dioxide and acids into alkaline solution is an example. Thus, an air pollution problem becomes a water pollution problem. Dust handling may involve some chemical treatment for stabilization or, in some cases, to recover valuable metals.

FIGURE 1.1 The daily metabolism of a city of 1,000,000 people (Wolman, 1965). Not all inputs and outputs are listed. (Photo credit: Pixabay.)
TABLE 1.1
Classification of Treatment Processes according to Their Suitability for Handling Different Substances
Classification of Treatment Processes according to Their Suitability for Handling Different Substances
An important part of many soil and groundwater remediation projects is separating the pollutants from the natural media so they can be treated using the processes that are common for wastewater and gaseous emissions.
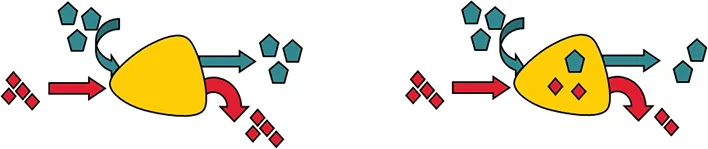
FIGURE 1.2 The law of conservation of mass says that all material entering a system must leave the system or accumulate within the system. The material balance (or mass balance) must account for all material flow.
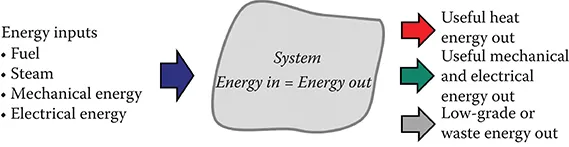
FIGURE 1.3 The law of conservation of energy says that all energy entering a system must accumulate in or leave the system. The energy balance is the engineer’s tool for making an accounting of all energy flow.
1.3 PROCESS ANALYSIS
Pollution prevention and control engineering involves the organization of artificial things to increase our level of health and happiness. As systems are created, in the engineer’s mind and on the drawing board, they must be evaluated. The quantities of all substances that flow through the system must be determined. The energy requirements must be calculated. These are the activities of process analysis. By analysis, we mean doing the process design calculation, not the laboratory process of identifying and quantifying chemical substances. The tools for doing the analysis are the material balance (Figure 1.2) and the energy balance (Figure 1.3). For details and example calculations, see Berthouex and Brown (2014).
1.4 PROCESS SYNTHESIS
The inventive part of design is process synthesis, through which we seek to satisfy the metabolism of our society with the least amount of damage to human health and environmental quality. Synthesis is the combining of diverse elements into a coherent whole that will produce the materials we consume without being wasteful of raw materials and with manufacturing methods that produce the smallest possible amounts of waste. And it means being clever about capturing and processing the waste products that are produced.
The synthesis of pollution prevention and control systems involves linking reactors and separation processes in support of each other.
Chemical and biochemical transformations are promoted and controlled in reactors in order to (1) upgrade an otherwise useless material to a material that is too valuable to waste, (2) destroy or inactivate a dangerous or harmful substance, and (3) facilitate the separation of one material from a bulk flow of other materials.
Chemical transformations are almost always integrated with separation processes. For example, a dissolved pollutant is converted to a solid particle by precipitation, and the solid is then separated from the liquid. The removed solid material may be a useful product, or it may be an undesirable substance, the removal of which raises the quality of the water so it can be safely discharged or reused.
Separation processes selectively remove one species of material from another (solids from a liquid, for example). This provides the means to concentrate the input to a reactor or to remove substances that would interfere with the reaction or damage the equipment. Separations are also used to remove from the reactor’s output any substances that have a high value, that are detrimental to downstream processes, or that cannot be safely discharged to the environment.
Figure 1.4 shows a simple reactor–separator system. Substance A is fed into a reactor and, after some reaction time, it is converted into a mixture of B and C. That the mixture may be saleable or useful, but more often value is added by separating the two materials. The desired product, B, must be purified by removing C. Material C, a by-product of the desired reaction, may have some value once it has been separated, or it may be an innocuous waste product or a waste that has a high cost of disposal.
Figure 1.5 shows a real treatment process that uses three chemical transformations and two separation processes to treat water that contains dissolved lead and sulfuric acid. Hydrated lime, Ca(OH)2, is added to neutraliz...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Contents
- Preface
- About the Authors
- Chapter 1 The Chemical Process Design Problem
- Chapter 2 Pollution and Pollutants
- Chapter 3 Organic Pollutants
- Chapter 4 Measuring Pollutants
- Chapter 5 Stoichiometry
- Chapter 6 Empirical Stoichiometry
- Chapter 7 Chemical Equilibrium for Acids and Bases
- Chapter 8 Precipitation Reactions
- Chapter 9 Oxidation–Reduction Reactions
- Chapter 10 Green Chemistry
- Appendix A: Atomic Numbers and Atomic Masses
- Appendix B: Equivalent Weights
- Appendix C: Computer Programs for Chemical Equilibrium
- References
- Index