![]()
1 | Cell Disruption Subbarayalu Ramalakshmi |
1.1 INTRODUCTION
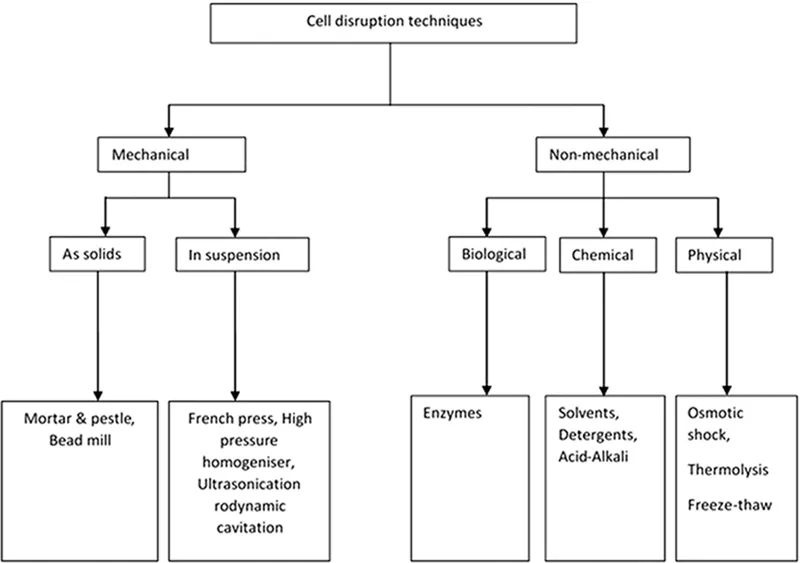
The product secreted during the process of fermentation is either intracellular, extracellular, or periplasmic. If the product is produced extracellularly, the desired product can be obtained from the liquid broth followed by further purification steps. On the other hand, if the product of interest is produced inside the cell (either cytoplasm or periplasm), it is indispensable to disrupt or disturb (in the case of periplasmic expression) the cell in order to extract the intracellular products. Cell disruption involves the pervasion or lysis of the cell that enhances the release of intracellular products (Harrison, 1991). Cells are highly robust in nature. The cell wall provides elasticity and mechanical strength to the cell and therefore provides resistance in order to withstand any sudden pressure changes in the external environment (Booth et al., 2007). Cellular disruption is not a separate procedure but depends on the upstream processes and considerably influences the downstream processes. Downstream operations account for about 70% of the total processing costs (Balasundaram et al., 2009). This requires an appropriate selection of the cell disruption method, which in turn affects the purification steps in downstream operations. Application of the disruption method depends highly on the nature and type of the cell. In case of bacterial cells, they are either gram negative or gram positive, and they differ in the composition of polysaccharide and peptidoglycan (Dmitriev et al., 2005; Silhavy et al., 2010). Yeast cells have a highly complicated extracellular organelle; they are also a rich source of various bioactive compounds, which require careful selection of the disruption method (Lipke and Ovalle, 1998; Okada et al., 2016). A mild disruption method is preferred for mammalian cells, which are easy to disrupt compared to plant cells and microalgae. Other factors such as low cost, maximum product release, ease extraction from the cell debris, and product stability govern the selection of disruption techniques. Cellular disruption methods are briefly categorized into mechanical and nonmechanical methods. Figure 1.1 shows the various existing cell disruption methods for the release of biological products (Harrison, 1991).
Certain measures should be taken before the cell disruption technique in order to increase the efficiency of the disintegration method. After the removal of cells from the culture medium, the products secreted extracellularly and also the media components should be reduced (Balasundaram et al., 2009; Harrison, 1991; Middelberg, 1995).
1.2 MECHANICAL METHODS
Mechanical cell disruption methods use both solid and liquid shear, and the cells are subjected to high pressure/agitation. Chemical agents or external reagents are not added in mechanical cell disruption, which is a chief advantage. Solid shear mainly relies on grinding and abrasion, while fluid shear relies on high pressure and velocity of fluid. Pestle-mortar was once a common method to disintegrate cells mechanically and is now rarely used. French press, ultrasonication, and glass bead disruption technology are currently used for small-scale purposes; high-pressure homogenizers (HPHs) and bead mills are widely used in for large-scale purposes. Mechanical methods of disruption have high efficiency and suits almost all types of cells. Most of the methods require cooling after the disruption processes. Heat generation, product degradation, and high cost are some of the drawbacks of mechanical lysis techniques (Goldberg, 2008; Lin and Cai, 2009).
FIGURE 1.1 Different cell disruption techniques. (From Harrison, S.T., Biotechnol. Adv., 9, 217–240, 1991.)
1.2.1 HIGH PRESSURE HOMOGENIZER (HPH)
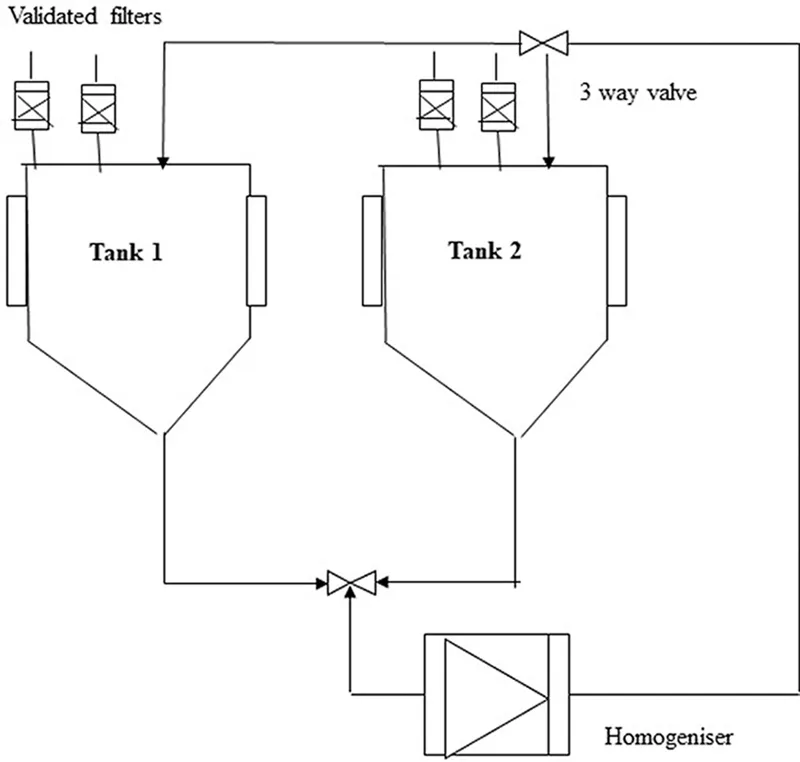
HPH is the extensively used method for lysing cells mechanically. Figure 1.2 shows the image of HPH used in industries (Middelberg et al., 1991). Though HPHs are commonly used for large-scale purposes, they are also available on smaller scales and can process 25–200 mL of sample volume (Lin and Cai, 2009). Cells present in the media after harvest are allowed to pass through an orifice (0.1–0.2 mm) where they are compressed by the application of high shear force. High shear, impact, and cavitation are the principles used in HPH. Two storage tanks are available in HPH that work alternatively and process the homogenate. Compressed cells are collected using a positive displacement pump.
FIGURE 1.2 High-pressure homogenizer used in industries. Tanks 1 and 2 process the cell suspension alternatively, and the cells are disrupted at the valve seats. (From Middelberg, A.P., Downstream Processing of Proteins, Springer, pp. 11–21, 2000.)
The amount of protein released by HPH is given by Equation (1.1):
where R is protein released; Rm is maximum protein available; P is pressure in MPa; N is the number of passes; K is the rate constant; and a is the pressure exponent (Augenstein et al., 1974; Middelberg, 2000; Middelberg et al., 1991).
High heat evolution and high energy consumption are common problems encountered in this method. An alternative method uses simultaneous emulsification and mixing in order to broaden the usage of HPH (Gall et al., 2016).
1.2.2 BEAD MILL
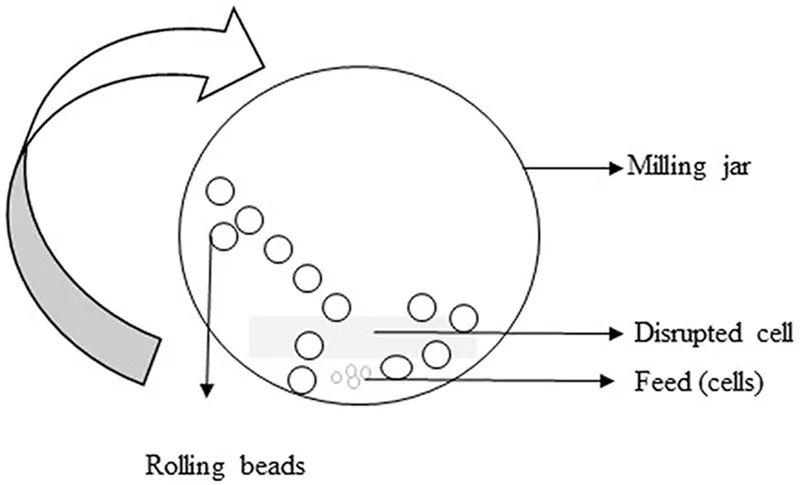
Bead mills are commonly used on a large scale, yet some are also employed in laboratories for disrupting cells. In this technique, cell suspension is mixed with glass, steel, or ceramic beads and agitated at high speed. High shear force is applied to the cells when they collide with the beads, which in turn disintegrate the cell membrane (Figure 1.3) (Pazesh et al., 2017). The type, size, and weight of the beads to be employed largely depends on the nature of the cells to be disrupted. Glass beads with a diameter greater than 0.5 m are suitable for yeast cell disruption, whereas those smaller than 0.5 m are suitable for bacterial cell disruption. The main governing parameters of cell disruption are bead diameter, number of beads, and agitator speed. The number of beads increases the efficiency of grinding; however, there are problems such as heat evolution, which causes product degradation. Bead shaking is applicable only at lab scale, and bead agitation is often used in industries (Chisti and Moo-Young, 1986; Harrison, 1991; Taskova et al., 2006). Shaking the glass beads with cells can disintegrate more than 60 E. coli samples in less than 30 min (Ramanan et al., 2008). Chances of contamination are encountered with this method, which is the main disadvantage.
FIGURE 1.3 Bead milling. Beads are mixed with the cells (feed), and the cells are lysed by the principle of grinding and abrasion. (From Pazesh, S., et al., Int. J. Pharm., 528, 215–227, 2017.)
1.2.3 ULTRASONICATION AND CAVITATION
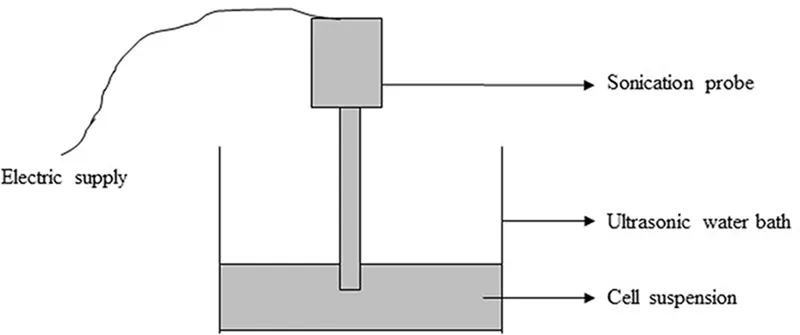
The process of cavitation uses the principle of sonochemistry. In this process, sound energy is generated electrically at a frequency ranging between 20 and 50 Hz. The sound energy travels through a probe that passes through the media solution or water placed in an ultrasonic bath. This process causes the formation of bubbles, which ultimately causes the cell membrane to rupture (Figure 1.4) (Bari et al., 2016). The technique has been used in various cells and in E.coli and Pichia pastoris (yeast) cells; the cells are processed in less than 0.3 and 1 sec, respectively. The overall rise in temperature during the process is 3.3°C, which is far better than electrical and thermolysis processes that causes product denature. The major challenge is that the process has to be optimized every time depending on the cell type and power input to the device (Tandiono et al., 2012).
Alternate methods are nitrogen cavitation and hydrodynamic cavitation. The physical stress is less in cavitation methods compared to ultrasonic method. Cells are placed in a pressure vessel and nitrogen free of oxygen is passed into the cells under high pressure (approximately 5500 KPa). Nitrogen bubbles are created, which causes the rupture of cell walls. It is best suited for fragile cell walls such as mammalian and plant cells and some bacterial cells, but not for fungi and yeast cells (Simpson, 2010). Hydrodynamic cavitation is an efficient method for the extraction of lipids in microalgae; it also causes less stress on the proteins and enzymes compared to the ultrasonic method. In hydrodynamic cavitation, the sample is passed through a small channel, which increases the velocity, thus causing the membrane to rupture and releasing the intracellular products (Save et al., 1997).
FIGURE 1.4 Ultrasonication process. A sonication probe is used to produce sonic waves for lysing the cells. (From Bari, S., et al., Ultrason. Sonochem., 31, 39–50, 2016.)
1.2.4 FRENCH PRESS
This is similar to HPH where application of high-pressure technique is employed. Yet this technique is used only for small-scale purposes (Goldberg, 2008). Initially, the cells are passed through a valve into a pump cylinder, after which they are allowed to pass through an annular gap. At this region, the pressure applied is 1500 bar. Then the cells a...