- 280 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Ablative and Non-ablative Facial Skin Rejuvenation
About this book
With the newer ablative and non-ablative techniques offering precise methods for improving photo-aged skin, facial skin rejuvenation is particularly popular. Ablative and Non-Ablative Facial Skin Rejuvenation discusses the various lasers, light sources, and radio-frequency devices currently used. Each chapter analyzes one of the available technolog
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
BIOLOGY OF COLLAGEN
KEY POINTS
- Collagen fibers comprise more than 70% of the dry weight of the dermis
- Type I collagen represents approximately 80% of the total collagen in the adult human dermis
- The varied thermal effects of heated collagen depend on the degree of temperature elevation
- Slight temperature elevation dissociates the intermolecular cross-links that stabilize the collagen triple-helix, resulting in collagen shrinkage
- Ablative and most non-ablative techniques will lead to an increase in Type I collagen
STRUCTURE
Collagen represents the main fibrillar component of connective tissue. Collagen also represents the major extracellular protein of the human body. The physiologic role of collagen fibers in the skin is to provide for the framework and mechanical strength of skin. This allows skin to serve as a protective organ against external trauma. Collagen fibers comprise more than 70% of the dry weight of the dermis. These fibers are composed of fibrils and microfibrils. Under electron microscopy, collagen fibers demonstrate a pattern of cross-striations appearing with a repeating periodicity approximately 70 nm apart. This regular banding pattern results from collagen molecules aligned in a quarter-stagger arrangement. Collagen molecules are composed of three polypeptides, known as chains, that are coiled around each other in a right-handed triple-helix. Each polypeptide chain has glycine distributed in a repeating Gly–X–Y sequence. The X position is often occupied by proline and the Y position is mostly occupied by hydroxyproline. The relatively high contents of these amino acids, and the repetitive positioning of glycine are necessary for the unique triple-helical conformation of the collagen molecule. The intermolecular cross-links unite the collagen molecule into a continuous polymeric network. This cross-linking is mediated by the copper-dependent enzyme lysyl oxidase and is inhibited by heat and photonic energy. Intermolecular cross-links give collagen unique properties of high tensile strength and elasticity.1
COLLAGEN TYPES
Collagen comprises a family of closely related yet genetically distinct proteins. The genetically distinct collagens can be divided into different classes based on the fiber architecture in tissues (Figure 1.1):
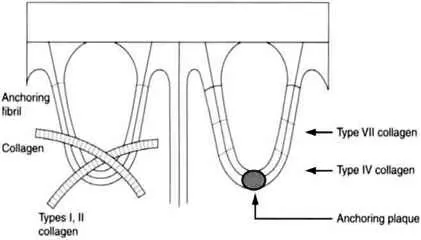
Figure 1.1. Location of various major collagen fibers in mature human skin
- Fibril-forming collagens, characterized by relatively large fibrils: collagen types I, II, III, V, XI.
- Interlacing network collagen: collagen type IV.
- Microfibril-forming collagen: collagen types VI, VII.
- Fibril-associated collagens with interrupted triple helices (FACIT): collagen types IX, XII, XIV.2
Type I collagen represents approximately 80% of the total collagen in the adult human dermis. Major fractions of type I collagen contains two identical a chains, α1(I) and a third chain, α2(I) which is different in amino acid composition. The chain composition of type I collagen is [α1(I)]2α2(I). Collagen molecules containing three identical α1(1) chains represent a minor fraction of collagen type I, called α1(I)3. Type I collagen is the most widely distributed and most extensively characterized form of collagen. It is responsible for the tensile strength of the human dermis. It represents the bulk of newly formed fully evolved collagen seen after treatment with ablative and most non-ablative dermal remodeling.
Type III collagen accounts for approximately 10% of the total collagen of adult human dermis. It predominates in human skin during embryonic life and is initially called fetal collagen. However, the ratio of type I to type III collagen in adult human skin is ~6:1 due to the accelerated synthesis of type I collagen during the early postnatal period.
Type IV collagen is predominantly present within the lamina densa of the dermal–epidermal junction. The presence of non-collagenous interruptions within the triple-helical domain of type IV collagen provides flexibility to the collagen molecule. In the lamina densa, type IV collagen forms a lattice pattern rather than the fibers characteristic of collagen types I–III. There are various a chains of type IV collagen identified as α1(IV), α2(IV), α3(IV), α4(IV), α5 (IV), and α6(IV).3 However, in human skin, collagen type IV is predominantly seen as the heterotrimer, [α1(IV)]2α2(IV).
Type V collagen represents less than 5% of the total collagen in human skin. However, migrating epidermal cells seen at the edge of an ablative resurfaced wound may produce type V collagen.4 In human skin, the predominant chain composition of type V collagen is [α1(V)] 2α2(V).
Type VI collagen consists of three distinct chains, α1(VI), α2(VI), and α3(VI), folded into a triple-helical domain of about 100 nm in length, with globular domains at both ends of the molecule.5 Type VI collagen forms a microfibrillar network rather than the broad fiber characteristic of types I and III collagens. The microfibrillar network of type VI collagen stabilizes the assembly of broad collagen fibers and basement membranes.
Type VII collagen is a major component of the anchoring fibrils that extend from the dermal– epidermal junction to the papillary dermis. The type VII collagen molecule has an unusually long triple-helical domain containing interchain disulfide bonds and a pepsin-sensitive, non-helical site close to the center of the molecule.6,7 It contains only one type of α chain, thus the chain composition of type VII collagen is [α1(VII)]3.
Type XII collagen contains more than one triple-helical domain separated by non-collagenous segments. Type XII collagen is in the group of FACIT collagens which form fibers in association with type I collagen.
Type XIV collagen, like other FACIT collagens, may also be oriented parallel to the surface of large collagen fibers.
Collagen Synthesis
Collagen genes, like most eukaryotic genes, are composed of exon and intron genes. Intron genes are non-coding DNA sequences of unknown function. The entire gene is transcribed into a precursor mRNA which undergoes post-transcriptional modifications, such as capping and polyadenylation. The mature mRNA undergoes a translation process in the cytoplasm of cells. The translation of collagen polypeptides occurs on the ribosomes of fibroblasts and related cells. The product of translation is called a prepro-a chain. This chain represents a precursor polypeptide of procollagen. Precursor polypeptides are then released into the cisternae of the rough endoplasmic reticulum. During this transmembrane transportation, the signal sequence of prepro-α chains is enzymatically cleaved, resulting in polypeptides called pro-α chains. Finally, these pro-α chains undergo post-translational modifications.
Synthesis of Hydroxyproline
Hydroxyproline represents about 10% of the amino acids in type I collagen. The presence of hydroxyproline is required for the final collagen triple-helical formation. This triple-helical conformation is the structure required for the normal secretion of procollagen molecules into the extracellular space. The formation of hydroxyproline is catalyzed by two separate enzymes, prolyl-4-hydroxylase and prolyl-3-hydroxylase. There are two isomeric forms of hydroxyproline in pro-α chains; trans-4-hydroxy-L-proline and trans-3-hydroxy-L-proline.8 The hydroxylation of procollagen polypeptides is initiated while the polypeptides are growing on the ribosomes and occurs only with prolyl residues residing in polypeptide pro-α chains. Hydroxyproline formation does not occur once the collagen substrate is in a final triple-helical conformation. Molecular oxygen, ferrous iron, α-ketoglutarate and a reducing agent are required as cofactors for hydroxyproline formation. Because reducing agents such as ascorbate and oxygen are required for the formation of hydroxyl groups in hydroxyproline, ascorbic acid deficiency and relative anoxia lead to poor wound healing and decreased tensile strength of the connetive tissue.9 The relative effect of ascorbic acid deficiency on post-laser collagen formation is unclear.
Synthesis of Hydroxylysine
Lysyl residues in pro-α chains are hydroxylated to hydroxylysine. An enzyme, lysyl hydroxylase, which requires molecular oxygen, ferrous iron, α-ketoglutarate, and ascorbate as cosubstrates, catalyzes hydroxylysine formation. As with hydroxylation of prolyl residues, only lysyl residues in peptide linkages are hydroxylated to hydroxylysine. Hydroxylysine formation begins while the polypeptide chains are growing on ribosomes and continues for some time after the release of polypeptides from the ribosomes. However, as is the situation with hydroxyproline, lysyl hydroxylase does not hydroxylate a collagen substrate that is in the triple-helical conformation. Thus, the rate of triple-helical formation of collagen can regulate the amount of synthesized hydroxylysine in collagen molecules. Hydroxylysyl residues in collagen molecules are required for the formation of collagen cross-links, and as an attachment site for glycosylated sugar residues.10,11
Glycosylation
Sugar residues are added to pro-α chains by a reaction called glycosylation. The sugar residues are linked to collagen polypeptides through the hydroxyl group of hydroxylysine. There are two glycosylation reactions, which attach galactosyl and glucogalactosyl residues to the collagen molecule. Collagen galactosyl-transferase and collagen glucosyl-transferase enzymes separately catalyze those two glycosylation reactions. These two enzymes of glycosylation require Mn2+ as a cofactor.10,11 The glycosylation reactions of pro-α chains begin after the synthesis of hydroxylysyl residues and terminate if the collagen substrate is in a triple-helical conformation.
After glycosylation, the three pro-α chains become associated. The association of the extensions of the individual pro-α chains facilitates folding of the collagenous portion of the polypeptides into a triple-helix. Interchain disulfide bonds are formed through the cysteine residues on the extension portion of the pro-α chain to link three pro-α chains together. The formation of the interchain disulfide bonds may have a role in the triple-helix formation of procollagen molecules. The rate of triple-helix formation of procollagen molecules varies between the genetically different types of procollagen. The rate of triple-helix formation limits some of the posttranslational modification reactions through the relative number of hydroxylysine and sugar residues in genetically distinct types of collagen. Hydroxylation, glycosylation and triple-helical formation occur within cisterna of the rough endoplasmic reticulum. Pro-α chains of collagen assembled on the membrane-bound ribosomes are translocated into this cellular compartment with microtubule involvement. After procollagen polypeptides are folded into a triple-helical conformation, procollagen molecules are then secreted in golgi vacuoles and transported into the extracellular space.10 Microtubule-disrupting agents such as colchicine delay the secretion of procollagen molecules in the extracellular space. The triple-helical conformation is required for the secretion of procollagen molecules. Newly synthesized but defective pro-α chains are degraded intracellularly before secretion. Genetically different types of procollagen molecules differ in their rate of secretion. In the extracellular space, procollagen molecules are converted to collagen by procollagen N-proteinase and procollagen C-proteinase. These two enzymes remove extension peptides on the collagen molecules. C-proteinase and N-proteinase are required for removal of the carboxy-terminal and amino-terminal extensions, respectively.11,12 The conversion of procollagen to collagen leads to subsequent collagen fiber formation. Failure to remove extension peptides, either the amino-terminal or carboxyterminal extensions, results in impaired tensile strength of collagen fibers in the skin. The collagen molecules spontaneously form collagen fibers after the removal of the extension peptides. The collagen molecules then undergo cross-linking to provide the tensile strength of collagen fibers. There are several forms of cross-links in collagen. The common forms are derived from lysyl or hydroxylysyl residues. These two residues undergo oxidative deamination, the first step in the cross-linking of collagen, catalyzed by lysyl oxidase. The enzymatic synthesis of aldehyde derivatives requires copper as a cofactor. Two synthesized aldehyde derivatives may form cross-links together or an aldehyde may form cross-links with an 8-amino group presented in unmodified lysine or hydroxylysine. This latter type of reaction is called a Shiff base-type of covalent cross-link. The collagen cross-links can be either intramolecular, occurring between two adjacent α-chains in the same collagen molecule, or intermolecular, linking with neighboring collagen molecules.
Thus the complicated process of collagen production is controlled at many different levels of synthesis and degradation. These levels include the: transcription and post-transcription level; translation and post-translation level; and degradation level. Any interference with collagen production would impact on the healing after any dermal remodeling technique.
Heat–Collagen Interaction
The varied thermal effects of heated collagen depend on the degree of temperature elevation. Slight temperature elevation dissociates the intermolecular cross-links that stabilize the collagen triple-helix, resulting in collagen shrinkage. However, the cross-links between collagen molecules remain intact. The heated polypeptide chains of collagen have a considerable but incomplete capacity to resume the original intrachain characteristics at a specific range of temperature elevation.13 Disruption of interpeptide bonds results in immediate shortening of collagen fibers to about one-third of their initial length. The shrinkage of collagen occurs along the long axis of collagen fibers and at a very specific temperature. The mechanism of collagen shrinkage is a transition between the triple-helical conformation and...
Table of contents
- COVER PAGE
- TITLE PAGE
- COPYRIGHT PAGE
- PREFACE
- 1: BIOLOGY OF COLLAGEN
- 2: CUTANEOUS WOUND HEALING
- 3: CARBON DIOXIDE LASER RESURFACING
- 4: ERBIUM: YAG LASER RESURFACING
- 5: COMBINED ERBIUM: YAG/CO LASER AND VARIABLE PULSED ERBIUM: YAG LASER
- 6: ELECTROSURGICAL SKIN RESURFACING
- 7: NON-ABLATIVE DERMAL REMODELING
- 8: COMPLICATIONS
- 9: MARKETING FACIAL SKIN REJUVENATION
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Ablative and Non-ablative Facial Skin Rejuvenation by David J. Goldberg in PDF and/or ePUB format, as well as other popular books in Medicine & Dermatology. We have over one million books available in our catalogue for you to explore.