
This is a test
- 344 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
UV light is one of a number of emerging non-thermal food processing technologies that can be used in a broad range of applications producing food products with longer shelf-life, more safe, and with higher nutritional quality. The new edition of Ultraviolet Light in Food Technology: Principles and Applications will present recent understanding of the fundamentals of UV light along with new applied knowledge that has accumulated during the 7 years since the first edition published in 2009. The new edition of the book will have 11 chapters including 2 new chapters--on chemical destruction with UV light and food plant safety—along with 6 chapters greatly expanded and updated.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Ultraviolet Light in Food Technology by Tatiana Koutchma in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Food Science. We have over one million books available in our catalogue for you to explore.
Information
1
Principles and Applications of UV Light Technology
CONTENTS
1.1 Introduction
1.2 Basic Principles of UV Light Technology
1.2.1 UV Light Generation
1.2.2 Gas Discharge
1.2.2.1 Pulsed Power Energization
1.2.3 Propagation of UV Light
1.2.3.1 Basic Principle of Photochemistry
1.2.3.2 Terms and Definitions
1.3 Applications Guidance of UV Technology in Food Processing
1.4 UV Disinfection of Surfaces
1.4.1 Food Contact Surfaces
1.4.2 Food Surfaces
1.4.2.1 Meat and Poultry Processing
1.4.2.2 Bakery Items
1.4.2.3 Shell Eggs
1.4.2.4 Fresh Produce
1.4.2.5 Fresh-Cut Produce
1.4.2.6 Postharvest UV Treatment of Grain
1.4.3 Pulsed Light for Food Surface Treatment
1.5 UV Light for Liquid Foods, Drinks, and Beverages
1.5.1 Juices
1.5.2 Wines
1.5.3 Liquid Sugars and Sweeteners
1.5.4 Liquid Egg Products
1.5.5 Dairy Products
1.5.6 Brewing Applications
1.5.7 Brines and Marinades
1.6 Conclusions
References
1.1 INTRODUCTION
There is a growing negative public reaction over chemical preservatives added to foods and drinks to extend their shelf-life and to protect against food-borne pathogens and spoilage microorganisms. To address the consumers’ demands for healthier food, the alternatives to current practices are being investigated. Among almost 30 emerging novel processing techniques, the ultraviolet (UV) light technology has been slowly taking its niche in food production as a truly non-thermal and non-chemical treatment. Even though the term “irradiation” is frequently used for this treatment, UV is also considered as a light. UV light is safe to use for foods; it is an ecologically clean technology because it is free of chemicals and waste effluents, and typically it does not produce by-products and can provide energy and cost-saving opportunities for food manufacturers. As a purely physical preservation method, UV light has a positive consumer image and is of interest to the food industry for microbial inactivation and destruction of undesirable chemicals. Although the use of UV light is well established for drinking water and wastewater treatment, air disinfection, and surface decontamination, it is still in limited use for food treatment. Recent advances in the science and engineering of UV light technology have demonstrated that UV treatment holds considerable promise in food processing as an alternative to traditional thermal processing for liquid foods such as juices, soft drinks, and beverages, post-lethality treatment for ready-to-eat (RTE) meals and shelf-life extension of fresh produce.
Surprisingly, little is known about the interaction of UV light with matter, especially with a complex food matrix, considering its importance. Radiative transfer covers all processes in which light or other electromagnetic energy is emitted or radiated with some of this energy transferred from one form to another, as it is in scattering and absorption. The particular type of interaction taking place in a liquid matrix can often be referred to as radiative transfer in a transparent, semi-transparent, or turbid medium. A turbid medium is defined as a substance which both scatters and absorbs part of the light falling on it. All translucent and opaque materials are, therefore, turbid media.
This chapter provides an overview of the fundamental scientific and technological principles of UV light treatments for foods. As a starting point to understanding UV light technology, recent information is provided on the nature of UV light, including the basic principles of UV light generation and propagation. The applications of UV light in food and drinks processing including pasteurization and shelf-life extension of juices and milk, post lethality treatment for meats, treatment of food contact surfaces and packaging materials, and to extension of the shelf-life of fresh produce are reviewed.
1.2 BASIC PRINCIPLES OF UV LIGHT TECHNOLOGY
Light is just one portion of the various electromagnetic waves traveling through space. The electromagnetic spectrum covers a broad range from radio waves with wavelength of a meter or more, down to x-rays with wavelength of less than a billionth of a meter (Figure 1.1).
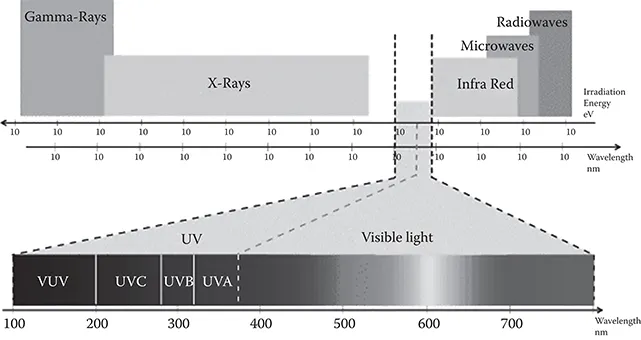
FIGURE 1.1 Electromagnetic radiation spectrum with a division of light spectrum on visible and UV light in the 3 different types ( www.zontec.net/files/uvSpectrum.gif).
Typically, the wavelength for UV irradiation ranges from 100 to 400 nm. This range may be further subdivided into UV-A diapason (315–400 nm) normally responsible for changes in human skin called tanning; UV-B (280–315 nm) that can cause skin burning and eventually lead to skin cancer; UV-C (200–280 nm) called the germicidal range since it effectively inactivates bacteria and viruses; and the vacuum UV range (100–200 nm) that can be absorbed by almost all substances and thus can be transmitted only in a vacuum. Short UV-C light is almost completely absorbed in air within a few hundred meters. When UV-C photons collide with oxygen atoms, the energy exchange causes the formation of ozone. UV-C is almost never observed in nature, since it is absorbed so quickly.
1.2.1 UV LIGHT GENERATION
Atoms and ions emit light when they change from a higher to a lower energy state. An atom and most ions consist of electrons orbiting a nucleus of protons and neutrons. The electrons in each orbital occupy a unique energy state, with the electrons closest to the nucleus having a lower energy and the electrons further away having a higher energy. When electrons make a transition from a higher energy, E2, to a lower energy, E1, a discrete amount of energy is released as photons of light. As first stated by Planck, each photon carries an energy E (J) described by Equation 1.1.
(1.1)
where h is Planck’s constant (6.23 × 10−34 J.s), c is the speed of light (2.998 × 108 m/s or m s–1), and λ is the wavelength of radiation (m). Energy levels of a given atom or ion are unique arising from the number of electrons, protons, and neutrons within that atom or ion, and their interaction with external force fields. As such, each element emits a unique spectrum of light. If the difference between energy levels is appropriate, the light emitted is UV.
A transition from a lower to a higher energy state requires an energy input. This energy may be derived from the collision of an atom with a photon of light of wavelength, λ, or by collision with other atoms, ions, or electrons. Energy transferred to the atom may result in an increase in the atom’s kinetic energy, the transfer of electron to a higher energy level, or the removal of an electron from the atom. Removal of an electron from the atom is termed ionization and results in a positively charged cation and a negatively charged free electron. Recombination of a free electron and a cation may result in the emission of light. Since the free electron and cation may have a range of kinetic energies, the wavelength of emitting light will be varying over a continuum or range. This wavelength (Equation 1.2) will be bound at one end by the ionization energy Eo of the atom and peak at a wavelength dependent on the temperature of the electrons and cations
(1.2)
For expressing the energy of a single photon, the unit of Joule (J) is a rather large unit.
Conventionally, the electronvolt (eV) is used. One eV is defined as the energy gained by an electron in passing through a potential difference of 1 Volt which equals 1.6 × 10–19 J. For photochemical purposes, the photon energy is often expressed in kilocalories per Einstein. Note that one Einstein is defined as being equal to Avogadro’s number (A) of photons = 6.02 × 1023 photons. Absorption of one Einstein can excite 1 mole of the absorbing substance (Equation 1.3)
(1.3)
where EE is energy per Einstein, A is the Avogadro’s number.
The photon may be viewed as the smallest discrete unit of radiation energy. Across the electromagnetic spectrum in a wide range of wavelengths, corresponding photon energies exist as shown in Table 1.1.
TABLE 1.1
Spectrum of electromagnetic radiation and corresponding photon energies
| Radiation type | Wavelength (nm) | Photon energy (kJ/Einstein) |
|---|---|---|
| Gamma rays | <0.1 | >106 |
| X-rays | ~0.1–50 | ~106–2,400 |
| UV light | ~50–400 | ~2,400–300 |
| Vacuum | ~50–200 | 2,400–600 |
| C | 200–270 | 600–440 |
| B | 270–330 | 440–360 |
| A | 330–400 | 360–300 |
| Visible | 400–700 | 300–170 |
| Infrared | 700–107 | 170–0.01 |
| Microwaves | ~107–108 | 0.01–0.001 |
| Radiowaves | ~108–1013 | 0.001–10−8 |
Radiation of the UV light and the adjacent visible spectral range as well as other less energetic types are summarily called non-ionizing radiation as opposed to ionizing radiation. The latter is represented in the electromagnetic spectrum by X-rays and gamma-rays. Other kinds of ionizing radiation consist of ionizing particles (beta-rays, alpha-rays, protons and electrons). Ionizing in contrast to non-ionizing radiation is capable of ionizing many atoms and molecules. Absorption of non-ionizing radiation, however, leads to electronic excitation of atoms and molecules. UV light at 253.7 nm has a radiant energy of 472.27 kJ/Einstein or 112.8 kcal/Einstein (one Einstein represents one mole of photons). It is theoretically possible for UV light at 253.7 nm to affect the O–H, C–C, C–H, C–N, H–N, and S–S bonds if it is absorbed.
1.2.2 GAS DISCHARGE
A gas discharge is a mixture of non-excited atoms, excited atoms, cations, and free electrons formed when a sufficiently high voltage is applied across a volume of gas. Light is emitted from the gas discharge at wavelengths dependent upon the elemental composition of the gas discharge, and the excitation, ionization, and kinetic energy of those elements. Gas discharges are responsible for the light emitted from UV lamps. When a voltage is applied, free electrons and ions present in the gas are accelerated by the electric field formed between two electrodes. With sufficient voltage, the electrons are accelerated to high kinetic energies. Collisions of the free electrons with atoms result in a transfer of energy to the atoms and if the energy is sufficient, the atoms are ionized. This ionization results in a rapid increase in the number of electrons and cations, with a corresponding increase in lamp current, and a drop in the voltage across the lamp.
Cations colliding with the electrodes cause electrons to be emitted. If sufficient electrons are emitted, a self-sustaining discharge occurs termed a glow discharge. With an increase in current, the larger fraction of each electrode will emit elect...
Table of contents
- Cover
- Half Title
- Series Page
- Title Page
- Copyright Page
- Table of Contents
- Series Preface
- Preface
- Series Editor
- About the Author
- Chapter 1. Principles and Applications of UV Light Technology
- Chapter 2. Sources of UV Light
- Chapter 3. Characterization of Foods Properties in Relation to UV Treatment
- Chapter 4. UV Light Microbial Inactivation in Foods
- Chapter 5. UV Light Processing Effects on Quality, Nutritional Content, and Sensory Attributes of Juices, Milk, and Beverages
- Chapter 6. UV Light for Fresh Produce and Grain
- Chapter 7. UV Process Calculations for Food Applications
- Chapter 8. UV Flow Systems for Treatment of Liquid Foods and Beverages
- Chapter 9. UV Process Validation
- Chapter 10. UV Applications for Food Plant Safety
- Chapter 11. UV Effects on Chemical Contaminants and Mycotoxins in Foods and Beverages
- Chapter 12. Current Status of UV Treatment of Foods in International Regulations
- Index