
eBook - ePub
Physiology for Anaesthesiologists
- 198 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Physiology for Anaesthesiologists
About this book
Reviewing all aspects of human physiology, Physiology for Anaesthesiologists emphasizes how those principles relate to the practice of anaesthesiology. It explains the various systems of the human body, from the central nervous system to the liver and kidneys, and how they interact with each other. The section on pain, for example, explains how the body warns of dysfunction in one or more systems. This volume provides a good general understanding of human physiology, giving readers the tools needed to apply physiological principles and knowledge to the clinical practice of anaesthesia. Targeted to anaesthesiologists and those in training, medical students in any field could benefit from the knowledge presented in this book.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Cardiovascular physiology
Pierre Foëx, Helen Higham
CARDIAC MUSCLE CONTRACTION
The cardiac cell
The cardiac cells (myocytes) are branched filament-like structures 10–20 μm in diameter and 50–100 μm long. Approximately every 2 μm in their longitudinal axis, transverse (T) tubules penetrate the cells. Transmembrane calcium channels are located in the transverse tubules in the immediate vicinity of calcium release channels (ryanodine channels), which are part of the membrane of the sarcoplasmic reticulum. The close proximity of these transmembrane channels facilitates the process of excitationcontraction coupling (Figure 1.1). Ion fluxes in and out of the myocytes, the sarcoplasmic reticulum, and the mitochondria are controlled by channels and pumps in their membranes. Sodium, calcium, and potassium ion fluxes are central to the process of depolarisation (Na+, Ca2+) excitation-contraction coupling (Ca2+), and repolarisation (K+).
Within the myocytes, the basic contractile unit is the sarcomere, composed of filaments of actin and myosin. The thick filament of myosin is formed by approximately 300 individual molecules of myosin and is about 1.5 μm long and 10–15 nm wide. Each molecule possesses a bilobed head. Half of the heads are oriented towards one end of the sarcomere and half towards the other. The thin filament of actin contains two helical chains of actin supported by tropomyosin molecules with troponin complexes placed at intervals of 38 nm. Troponin C, to which calcium binds, is part of a complex that includes troponin I (the inhibitory protein for the interaction of actin and myosin) and troponin T, which links the troponin complex to tropomyosin.
Contraction of the myocytes
Following depolarisation, calcium ions enter the myocytes during the plateau phase of the action potential. This causes a triggered release of calcium through the ryanodine channels of the sarcoplasmic reticulum. The rapid increase in myoplasmic calcium results in calcium becoming available to bind with troponin C. This, in turn, removes the inhibitory effect of troponin I on the interaction of actin and myosin. Hydrolysis of adenosine triphosphate (ATP) allows the thick and thin filaments to slide past each other.
The lateral projections of the myosin filaments, with their articulated heads attached to an arm that is capable of sustaining tension, give the sliding of the filaments an oar-like motion. When the arms abduct, the heads come closer to the actin filament and crossbridges are formed at the level of active sites. Head rotation pulls on the arm and causes the actin filament to move with respect to the myosin filament (Figure 1.2). This results in both force development and shortening. The total force developed in this process is a function of the number of cross-bridges formed. Formation of each crossbridge requires one molecule of ATP. In addition to initiating the contractile cycle, calcium ions alter the kinetics of cross-bridge formation by altering the myosin ATPase activity: this is the fundamental mechanism of increases in the rate of cross-bridge formation, i.e. increases in contractility.
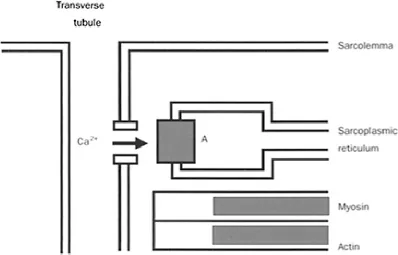
Figure 1.1 Schematic representation of a calcium channel located in a transverse tubule in the immediate vicinity of a ryanodine receptor (A) of the sarcoplasmic reticulum.
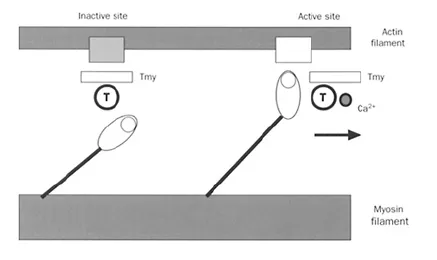
Figure 1.2 Diagrammatic representation of actin and myosin filaments. The lateral projections of the rotating heads of myosin can attach to the actin filaments when inactive sites become active. This occurs when calcium ions become attached to tropocin (T), thus causing a change in the configuration of tropomyosin (Tmy), allowing the interaction of actin and myosin.
THE CARDIAC CYCLE
Each cardiac cycle consists of a period of contraction (systole) followed by a period of relaxation (diastole) (Figure 1.3). Atrial systole is initiated by the spontaneous generation of an action potential in the sino-atrial node. After a delay due to the slow conduction velocity of the atrioventricular node, the ventricles are activated and systole begins. In its early phase, the mitral, tricuspid, aortic, and pulmonary valves are closed and the ventricles contract isovolumically. Once the ventricular pressures exceed the aortic and pulmonary pressures, ejection starts. It will cease once the ventricular pressures become smaller than the aortic and pulmonary pressures. Ventricular pressures then decrease without change in volume because the atrioventricular valves are also closed: relaxation is isovolumic. Once the ventricular pressures are smaller than the atrial pressures, the atrioventricular valves open and filling starts. Note that ventricular emptying and ventricular filling are fastest at the beginning of ejection and filling respectively.
Ventricular filling, after its early rapid phase, becomes much slower or even stops (diastasis), until it is completed by atrial contraction. Opening and, respectively, closure of the aortic and pulmonary valves are responsible for the first and second heart sounds.
For each cardiac cycle, the instantaneous relationship between ventricular pressure and volume forms a pressure-volume loop. The loop has four segments representing isovolumic contraction, ejection, isovolumic relaxation, and filling. The width of the loop represents the stroke volume; its surface area represents the stroke work. For a given ventricle and for a constant inotropic state, the loops are bounded by the end-diastolic pressure-volume relationship (an expression of ventricular stiffness), and the endsystolic pressure-volume relationship, which represents the contractile state of the myocardium. Positive inotropic interventions increase, while negative inotropy decreases the slope of the end-systolic pressure-volume relationship, also termed the maximum elastance.
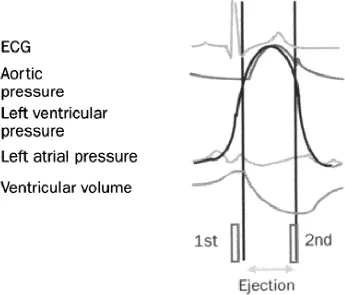
Figure 1.3 Various waveforms that characterise the cardiac cycle: electrocardiogram (ECG), aortic pressure, left ventricular pressure, left atrial pressure, and left ventricular volume, with schematic representation of the first and second heart sounds.
REGULATION OF CARDIAC FUNCTION
Performance of the heart as a muscle and as a pump is influenced by three major determinants: resistance to contraction/ejection (the afterload), initial fibre length/ventricular filling (the preload), and changes in the contractile state of the cardiac muscle (contractility, inotropy). In the face of ventricular hypertrophy and/or ischaemia, ventricular compliance (or its inverse, ventricular stiffness) becomes an important determinant of pump function.
Preload
The resting length of the sarcomeres is the true preload of the cardiac muscle: its relationship to the force developed during contraction is the cellular basis of Starling’s law of the heart. Maximum force can be developed when the resting sarcomere length is 2.2 μm. At greater sarcomere lengths, the scope for cross-bridge formation is reduced because the actin and myosin filaments are too far apart. At smaller sarcomere lengths, the filaments are farther apart rather than buckled; this reduces the number of crossbridges that can be formed.
In the intact heart, the end-diastolic pressure of the ventricles is commonly used as representing the preload. For the left ventricle, the pulmonary artery occluded pressure (pulmonary wedge pressure) is generally used to represent the preload. Changes in preload are associated with changes in stroke volume, stroke work, and cardiac output. The relationship between cardiac output and end-diastolic pressure is curvilinear (ventricular function curve). This is the expression of Starling’s law of the heart (Figure 1.4).
Contractility
In the isolated heart muscle and in the intact heart, for a given preload, force development and stroke volume can be increased by positive or decreased by negative inotropic interventions, resulting in upwards or downwards shifts of the ventricular function curve (Figure 1.4).
Changes in the inotropic state of the myocardium ultimately result from either an increase in myoplasmic calcium or increased sensitivity of the contractile apparatus to calcium. Increases in myoplasmic calcium result from receptor-mediated increases in cyclic adenosine monophosphate (cAMP) (β-adrenoceptor stimulation) or reduced breakdown of cAMP (phosphodiesterase inhibitors).
Increased sensitivity to calcium is an important factor in the preload dependence of force development, suggesting that the degree of actin-myosin overlap may not be the critical factor in Starling’s law of the heart. Thus the effects of preload and contractility on cardiac muscle performance may relate to the same mechanism, i.e. a reduced inhibition of actin-myosin interaction due to changes in troponin I.
CONTROL OF CARDIAC OUTPUT
Cardiac output (the product of heart rate and stroke volume) averages 5.0 1/min in a resting 70 kg supine man. It can increase fivefold with strenuous exercise. Variations in cardiac output can be produced by changes in heart rate or stroke volume. Heart rate is controlled primarily by the autonomic nervous system: sympathetic stimulation increases while parasympathetic stimulation decreases the cardiac rate. The stroke volume is determined by three main factors: preload, afterload, and contractility. Preload and afterload are highly sensitive to changes in metabolic requirements (whole-body oxygen consumption). Where local regulation causes peripheral or splanchnic vasodilatation, venous return is increased so that the preload of the ventricles is augmented; at the same time, resistance to left ventricular ejection is reduced, allowing stroke volume to increase. Preload, afterload, and contractility are influenced by the autonomic nervous system. α Adrenergic stimulation causes arteriolar and venoconstriction. β Adrenoceptor stimulation increases contractility. Parasympathetic stimulation decreases vascular resistance and depresses cardiac contractility.
RHYTHMICITY OF THE HEART
Cardiac cells that are capable of developing spontaneous diastolic depolarisation are called pacemaker cells; they generate the cardiac rhythm. Normally, the dominant pacemaker of the heart is the sinus node. In adults, it fires at a rate of 60–100 beats/min. Cells capable of developing spontaneous diastolic depolarisation are also found in specialised fibres in the atria, the atrioventricular node, and the His-Purkinje system. These cells exhibit a slower rate of spontaneous depolarisation than the sinus node.
Spontaneous depolarisation of pacemaker cells results from ionic currents across the cell membrane, including a non-specific inward current followed by a transient inward calcium current carried by T-type calcium channels. Depolarisation spreads from the sino-atrial node to the atrial tissue, initiating atrial contraction, and towards the atrioventricular node where conduction is slow so that activation of the ventricles through the bundle of this is delayed, allowing atria to contribute to ventricular filling before ventricular contraction starts.
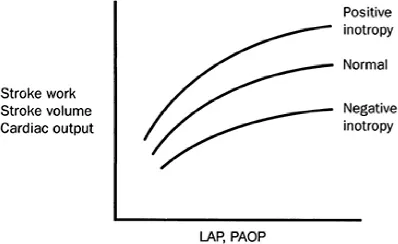
Figure 1.4 Left ventricular function curves demonstrating the upward and downward shifts caused by an increase, respectively decrease, of contractility. LAP, left atrial pressure; PAOP, pulmonary capillary wedge pressure.
The action potential consists of a transient, local trans-sarcolemmal depolarising current that raises the transmembrane potential from its resting value (-80 to -90 mV) to a slightly positive value (Figure 1.5). In contractile cells, the earliest and largest component of membrane depolarisation is the rapid inward influx of sodium ions. The resting potential is established and maintained by the trans-sarcolemmal Na+/K+-ATPase that pumps sodium ions out of the cytoplasm.
In contractile cells, calcium influx through more slowly opening channels produces the plateau phase of the action potential, while potassium efflux through two types of potassium channels produces both the inward rectifier current (IKI) and the delayed rectifier current (IK). By contrast, in rhythmically discharging cells (pacemaker cells), the resting membrane potential declines to the threshold level at which depolarisation, carried largely by calcium ions, occurs. Spontaneous depolarisation is due to calcium entry through T-type channels, while acute depolarisation is due to calcium entry through Ltype channels. At the peak of depolarisation, potassium channels open and repolarisation is initiated, so there is no plateau phase (Figure 1.5).
Control of heart rate by the autonomic nervous system results from changes in the speed of spontaneous depolaris...
Table of contents
- Cover Page
- Title Page
- Copyright Page
- Contributors
- Series Preface
- 1: Cardiovascular physiology
- 2: Respiratory physiology
- 3: Neuromuscular physiology
- 4: Gastrointestinal physiology
- 5: Mechanisms of pain
- 6: Red blood cells, haemostasis, and white blood cells
- 7: The Endocrine System
- 8: Renal physiology
- 9: Physiology of the liver
- 10: Immunology
- 11: Physiology of the nervous system
- 12: Cell structure, function, and regulation
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Physiology for Anaesthesiologists by J.P. Howard Fee, James G. Bovill, J.P. Howard Fee,James G. Bovill in PDF and/or ePUB format, as well as other popular books in Medicina & Anatomía. We have over one million books available in our catalogue for you to explore.