
eBook - ePub
Comparative Genomics and Proteomics in Drug Discovery
Vol 58
This is a test
- 164 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
Comparative Genomics and Proteomics in Drug Discovery gives an overview of how emerging genomic and proteomic technologies are making significant contributions to global drug discovery programs, and in particular the key role that comparative genomics and proteomics play within this strategy. Each chapter is written by respected authorities, with hands-on experience, from both academic and pharmaceutical backgrounds.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Comparative Genomics and Proteomics in Drug Discovery by John Parrington,Kevin Coward in PDF and/or ePUB format, as well as other popular books in Medicina & Farmacología. We have over one million books available in our catalogue for you to explore.
Information
1
Comparative genomics and drug discovery in trypanosomatids
Trypanosomatids are parasitic protozoa responsible for a range of diseases, including African sleeping sickness, Chagas disease and leishmaniasis. They infect millions of humans worldwide and are responsible for thousands of deaths every year. There are no vaccines and the available drugs, most of them developed many decades ago, have multiple often toxic side-effects. The genome sequences of three of these trypanosomatids (Trypanosoma brucei, T. cruzi and Leishmania major) have been published recently. This allows researchers to compare these genomes and search for common, as well as species-specific, features in order to identify putative drug targets.
In this chapter we describe the main characteristics of trypanosomatids at the genomic level as well as the most relevant findings in terms of comparative genomics. We review current approaches to drug discovery in trypanosomatids that use information derived from the genome project and evaluate the future potential of the genome information available.
1 Introduction
1.1 General characteristics of trypanosomatids
1.1.1 Taxonomy
The family Trypanosomatidae belongs to the protist order Kinetoplastida and includes a wide range of flagellated protozoa exhibiting different shapes, life cycles, geographical distributions and cellular, molecular and genetic characteristics. Kinetoplastids are among the most ancient eukaryotes and, according to rRNA studies, they have rRNA lineage extending further back than those of animals, plants or fungi (Fernandes et al., 1993; Beverley, 1996).
As members of this order, all the trypanosomatids present a unique and characteristic structure called the kinetoplast, a network of mitochondrial DNA located within the typical single long mitochondrion of these organisms, which is associated with the basal body of the flagellum. It contains two different kinds of concatenated circular DNA molecules called maxicircles and minicircles. Further description of the mitochondrial DNA is presented in Section 2.1.
Two of the genera included in the Trypanosomatidae family are Trypanosoma and Leishmania. These two genera appeared long ago and some studies support the idea that their common ancestor disappeared between 400 and 600 million years ago (Stevens and Gibson, 1999; Overath et al., 2001; Stevens et al., 2001). Apart from the presence of the kinetoplast, members of these genera present other unique characteristics such as eukaryotic polycistronic transcription, presence of unique organelles (e.g. glycosomes), distinctive metabolic pathways as well as other significant characteristics, not unique to trypanosomatids, such as antigenic variation of surface glycoproteins and expansion/contraction of subtelomeric regions.
Trypanosomatids have in common that they are all parasitic flagellated protozoa. Among them are the causative agents for several diseases; some of the most representative and well studied are Trypanosoma brucei, which causes African trypanosomiasis or sleeping sickness, Trypanosoma cruzi, which produces American human trypanosomiasis or Chagas disease in South America and Leishmania major, responsible for cutaneous leishmaniasis.
1.1.2 Ecology and geographical distribution
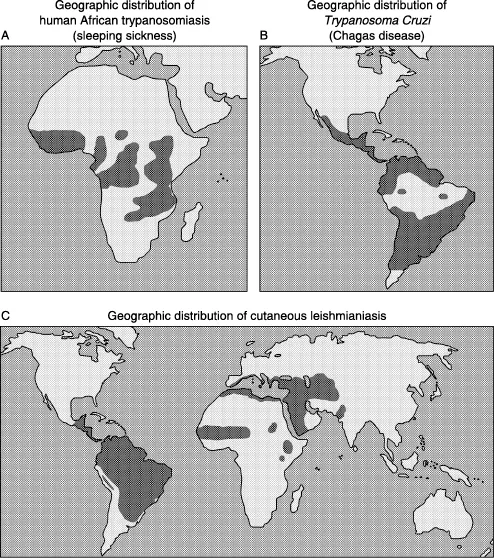
The three species mentioned above require a vector to infect new hosts (mammals, including humans), therefore their geographical distribution and ecology is driven by distribution and ecology of their vectors and hosts. In the case of T. brucei the vector is the tsetse fly (genus Glossina) which is found in Sub-Saharan Africa. Tsetse flies live in habitats that provide shade for developing puparia and resting sites for the adults. Temperature and therefore climate and altitude are determining factors for the development of tsetse fly populations (Hargrove, 2004; Rogers and Robinson, 2004). Figure 1A shows the distribution of human sleeping sickness in Africa.
Despite differences in its life cycle (see below), T. cruzi shares some common features with T. brucei. For instance, it is transmitted from host to host by insects, not by a fly but by members of the order Hemiptera, known as kissing or ‘assassin’ bugs. Geographical distribution of T. cruzi covers areas from southern South America to southern North America (Figure 1B).
Geographical distribution of Leishmania is wider compared to Trypanosoma species as it can be found in eastern and northern Africa, Central and South America, the Middle East, parts of Asia and even in southern Europe. Depending on the type of vector its habitat ranges from rainforest to arid regions. In general, Leishmania is transmitted to humans through the bite of sand flies. Figure 1C shows world distribution of L. major.
According to The World Health Organization members of the Trypanosomatidae family causing human diseases infect between 15 and 20 million people worldwide (most of whom do not have access to treatment) and are responsible for the death of hundreds of thousands of people each year (WHO, 2002). Most of these infections and deaths take place in poor countries where these diseases have a very important social and economic impact. A good example of this is the case of the genus Trypanosoma. Apart from the causative agent responsible for human African trypanosomiasis, this genus includes the species T. vivax and T. congolense, which are unable to infect humans but are responsible for a similar sickness in mammals (mainly in cattle and other livestock) known as ‘nagana’. As a result of this, the following observation has been made: on the approximately 7–10 million Km2 of land infected by the tsetse fly in Africa only 20 million cattle are raised, however, that land could support under other circumstances up to 140 million cattle and increase meat production by 1.5 millions tons (Molyneux and Ashford, 1983).
1.2 Life cycles
Life cycles of trypanosomatids are generally very complex and involve more than one host (the vector and a mammal). Here we will describe briefly the life cycle of T. brucei, T. cruzi and L. major, emphasizing the most relevant events that take place during these cycles.
1.2.1 Trypanosoma brucei
There are four main forms or stages in the life cycle of T. brucei: metacyclic form, bloodstream form, procyclic form and epimastigote. When an infected tsetse fly feeds, the parasite is injected into the mammalian host as a non-dividing metacyclic trypomastigote form. At this stage the parasites are expressing a repertoire of metacyclic variant surface glycoproteins (VSGs) that coat the surface of the parasite, and contain a central vesicular nucleus, a posterior kinetoplast and basal body, with a flagellum running anteriorly along an undulating membrane. As soon as the parasite is in the bloodstream it undergoes a series of morphological and molecular changes to produce the slender bloodstream form of the parasite. This form replicates asexually by binary fission and the flagellum extends beyond the anterior end.
An important molecular event that takes place at this stage is the expression of a new type of VSG. The parasite is able to vary the VSG coat as it replicates, to evade the host immune system (Borst, 2002; Pays et al., 2004). At any one time each cell expresses only one specific VSG (about 107 copies per cell forming a dense coat throughout the cell surface) but after a period of time (around 100 divisions) the parasite starts to express a different VSG. Therefore, the host immune system needs to produce new antibodies against the new VSG. This process is repeated to produce a series of parasitaemic waves in the host. This strategy allows the parasite to remain extracellular during its life cycle in the host mammal bloodstream. Eventually, in the last stages of the sickness the parasites also enter the central nervous system (see Section 1.3).
In the bloodstream a transition takes place in a subset of slender form parasites to produce the stumpy form. This form is non-proliferating and appears at the peaks of parasitaemic waves, is preadapted to survival in the tsetse fly but is still covered by VSGs. A tsetse fly becomes infected by taking up stumpy forms when feeding on an infected mammal. In the fly the parasite migrates to the posterior part of the midgut and differentiates into a proliferating form called the procyclic, which divides by binary fission. At this stage the kinetoplast has moved to a region between the nucleus and the posterior end of the cell and the cellular surface has lost its VSG coat and is now covered by a coat of the insect stage-specific protein procyclin. Then the parasite leaves the midgut and migrates to the salivary glands where it differentiates into epimastigotes that continue dividing by binary fission. Epimastigotes have a prenuclear kinetoplast and basal body. Finally, epimastigotes differentiate into metacyclics, ready to be transferred to a new host during the next fly bite. The cycle in the fly takes around three weeks and genetic exchange can occur but is not obligatory.
1.2.2 Trypanosoma cruzi
The mammalian host becomes infected during or after the blood meal of an infected triatomine insect vector. These insects release metacyclic trypomastigotes in the faeces, which enter the host through the bite wound or mucosal membranes. Unlike T. brucei, once inside the host T. cruzi invades cells in different tissues and differentiates into amastigotes, losing the flagellum. Since they are intracellular they are less exposed to the host immune system and do not apply the dramatic phenomenon of antigenic variation. Amastigotes divide by binary fission, differentiate into trypomastigotes and are released in the bloodstream again. In contrast to T. brucei, these bloodstream trypomastigotes do not replicate.
The insect vector is infected when it feeds on blood of infected mammals with circulating parasites. The parasites transform into epimastigotes in the vector’s midgut where they divide until they transform into infective metacyclic trypomastigotes in the hindgut.
1.2.3 Leishmania major
In all species of Leishmania, there are two main stages: the promastigote and the amastigote. As described for Trypanosoma species its life cycle includes a mammalian host and a vector, in this case a sandfly.
The mammalian host becomes infected with promastigotes during the bite of an infected sandfly. Once inside the host, the parasites are phagocytosed by macrophages where they transform into amastigotes and divide rapidly. The very high division rate shown by these amastigot...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Contributors
- Preface
- 1. Comparative genomics and drug discovery in trypanosomatids
- 2. The practical implications of comparative kinetoplastid genomics
- 3. The relevance of host genes in malaria
- 4. Nicotinic acetylcholine receptors as drug/chemical targets, contributions from comparative genomics, forward and reverse genetics
- 5. Discovery of novel sodium channel inhibitors: a gene family-based approach
- 6. ‘Omics’ in translational medicine: are they lost in translation?
- 7. Drug-target discovery in silico: using the web to identify novel molecular targets for drug action
- Index