C
CAM (D&T)
A cam mechanism converts a rotary motion to a linear movement (see crank forthis in reverse). The cam itself is an off-centre or specially shaped wheel that transmits its movement to a follower, which is the name for the arm that follows the movement of the cam. The follower may be attached to the cam by a split pin, or it may only rest on the cam, held in place by guides. Springs may push the follower against the cam or gravity may keep the follower in contact.
CARBON CYCLE
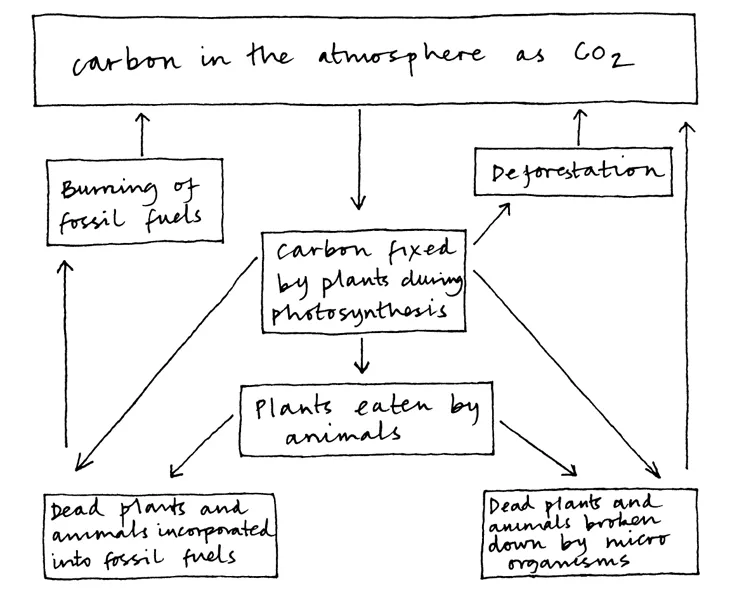
An understanding of the carbon cycle helps us to understand climate change more completely. Figure C.1 illustrates the terrestrial part of the cycle. Carbon in the air (as carbon dioxide) becomes incorporated into plants through the process of photosynthesis. This carbon passes through the food chain, and at each level the bodies of dead plants and animals are broken down by micro-organisms which release the carbon back into the atmosphere in the form of carbon dioxide. The carbon load in the atmosphere remains the same providing the amount released by decay is balanced by the amount taken up by new plant growth. However, deforestation upsets the balance, releasing carbon held in trees. Other man-made problems are changing the balance too. About 300 million years ago Britain occupied an area of the planet where plants died in an environment that inhibited their decay so that the carbon from them was preserved in the form of fossil fuels. The carbon remained trapped in rocks until very recently, when humankind’s need for energy led to coal, oil and gas being extracted and burnt (see burning). This has released large quantities of carbon dioxide into the atmosphere. Many scientists believe that carbon dioxide (along with other greenhouse gases) traps the sun’s energy on the surface of the Earth and its lower atmosphere, leading to climate change.
CELL (ELECTRICAL)
– see battery (cell)
CHARGE (ELECTRICAL)
Charge can be thought of as the ‘amount’ of electricity in a particular place. It is measured in coulombs (C) after the French scientist Charles Augustin de Coulomb (1736–1806). Most of the tiny particles that make up the atoms and molecules in all the things around us carry an electric charge. Protons at the centre of atoms are positively charged, whilst the electrons that orbit around them are negatively charged. Since there are usually the same number of protons and electrons in any atom and theircharges are equivalent they usually cancel each otherout,
so the overall electrical charge is zero. However, when electrons move from one place to another – eitherthrough the chemical reaction in a battery or by being ‘rubbed off’ an insulating material (see insulator) – this causes a separation of charge. Wherever the electrons have gone to, they tend to outnumber the protons, so there is a net negative charge. Conversely, wherever the electrons have been removed from, the protons will be left in the majority so that area will have a net positive charge. This separation of charge creates an attractive force (see attraction) between the oppositely charged particles; the electrons are attracted to the positively charged protons, which can result in the sparks or electric shocks we sometimes experience getting out of a car, lightning, or the flow of electric current around a circuit.
CHARTERED SCIENCE TEACHER
Chartered Science Teacher (CSciTeach) status is available to primary as well as secondary school teachers and to other science educators. It is a professional qualification which recognises the professional standing of an individual working in that field and carries a chartered designation in line with other awards, such as Char-tered Accountant or chartered Surveyor. The award is made under powers granted by the Privy Council. Chartered science teachers are required to be members of the Association for Science Education (ASE) and to have at least four years’ experience in teaching science. Typically applicants are required to have a Master’s level qualification in education and an honours degree with a minimum of 50 per cent science content, but it may be possible to demonstrate an equivalent level of expertise through other experience. The ASE is the awarding body for chartered Science Teacher status.
CHEMICAL CHANGES TO MATERIALS
During chemical changes, new substances are produced. Such changes can be accelerated orcaused by heat, and are usually permanent. In cookery, for example, a hard-boiled egg cannot be made soft again by cooling it, and dough baked in an oven and made into bread cannot be returned to flour and water. Neithercan clay once it has been fired in a kiln be returned to its original state. During chemical changes, a chemical reaction takes place in which the particles involved undergo significant changes.
Two of the commonest processes that lead to chemical changes are burning and corrosion. In the example of a candle burning, new chemical bonds (see bond-ing) are formed between carbon atoms and oxygen atoms to make carbon dioxide molecules. The carbon atoms are firmly bound to the oxygen atoms inside the carbon dioxide molecules and cannot easily be separated. A new compound has been created. During a chemical change no new matter is created or destroyed. The same numberof atoms exist but they have been rearranged – the mass has been conserved (see conservation of matter). When an iron nail corrodes, the iron reacts with oxygen in the airto create a coating of iron oxide or rust. Decay is another form of permanent change often brought about by the actions of micro-organisms. If a material decays in this way it is biodegradable (see biodegradability).
A starting point for teaching chemical changes could involve presenting a particular material or object to children. Their ideas could be elicited about how it might change. Such items could be an ice cube or ice balloon, a puddle of water, a burning candle, chocolate, perfume, clay, an egg, a rubber band, sugar, a rock or a coin. Key questions to ask when eliciting children’s understanding of chemical changes (as opposed to physical changes to materials) include: How can we change the material? Couldyou get the material to change back? Would any new materials be made?
From this starting point children’s understanding can be developed through cooking, through studying materials that rust and through observation of objects being burnt (most obviously candles). Attention can be focused on the material that is being changed, what new products are formed and the process that produces these new products.
CHEMICAL PROPERTIES OF MATERIALS
A property of materials that is determined by the way a material reacts when in contact with another material. An example of a chemical property is reactivity, which is a measure of a material’s inertness or resistance to change in any given reaction. Materials which are more reactive take part in chemical reactions more readily. Corrosion is a specific type of chemical reaction that can be studied in the primary school. Iron metal reacts readily with oxygen and water to form rust, whereas copper cor-rodes much more slowly. There are a number of factors which affect the rate of corrosion and these can be investigated (see investigation) in the primary classroom.
CHROMATOGRAPHY
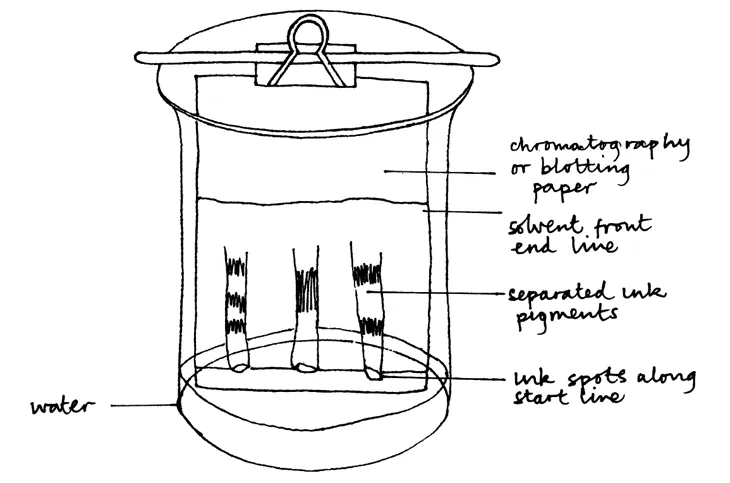
Chromatography is a technique which enables complex mixtures of chemicals in solution to be separated. Solutes (such as sugar and salt) dissolved in the same solvent (such as water) are difficult to separate; however, it is possible to separate some mixtures of solutes into thei rcomponents by paper chromatography (see Figure C.2). In the primary classroom, possibly the commonest investigation involving chromatography is to separate out the coloured dyes offelt-tipped pens (maybe as part of a ‘forensic’ test to determine who wrote the mystery note!). Put a spot of each pen’s ink on blotting paper and drip water on to it. As the water spreads out it carries the inks different distances. It is possible to separate inks into constituent colours by chromatography because the solvent soaks through the filter or blotting paper and the coloured inks separate out as they travel at different rates across the paper. Dark blue, brown and black inks work particularly well, sometimes revealing a range of other colours.
CIRCUIT (ELECTRICAL)
An electrical circuit consists of a loop of conducting material (wire), a source of electrical energy (battery/cell) and usually some other type of electrical component which transfers the electrical energy into another form (e.g. a lamp, buzzer or motor). Electrical current requires a complete circuit to flow, since all the tiny particles (electrons) move in the same direction. This is something children struggle to grasp; even if they have made simple circuits with batteries, wires and bulbs it still doesn’t seem to make sense that you need wires going to and from the battery. This confusion results
from a source-sink model which many people carry around in their heads. In this model, the battery/cell is the ‘source’ of electricity, which flows down the...