
- 368 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Practical Hydraulics Handbook
About this book
The Second Edition of the Practical Hydraulics Handbook is a must for all those who work with water utility systems. Presented in workbook format and emphasizing practical applications, this Handbook is perfect for hydraulic engineers, technicians, operating personnel, supervisors, managers, consultants, and students.
The exceptionally well-organized chapters include information on pressurized systems and open channel flow, principles of energy and force, flow calculations and measurement, pumps, and pumping applications.
This latest edition of the Practical Hydraulics Handbook includes new exercises at the end of each chapter and detailed solutions to selected exercises. The well-chosen exercises allow readers to practice applications of the theory and to test their knowledge of the material. The solutions provide guidance and problem-solving techniques that can be used both in the field and in the lab. Reference tables are also provided for calculations of friction loss, velocity, pipe fullness, well drawdown, English/metric conversions, power, and metered flow. These tables make calculations easier and minimize the chance for error.
In this new edition of Practical Hydraulics Handbook, all of the major principles and calculations dealing with the hydraulics of water systems are covered, and new and expanded material has been added.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Mass, Density and Displacement
mass & weight
Mass is the quantity of matter that a substance contains. It is a basic property of the substance, and is constant, regardless of location. Mass is most frequently registered in units of grams, or pounds; it is measured with an analytical balance which compares it against a known mass.
Weight is the effect of the force of gravity upon a substance, and is measured with a scale. A block of steel which weighs ten pounds on earth, will weigh much less on the moon, where gravitational forces are less. Its mass, however, will be the same in both places.
Since we are dealing only with earthly water systems, for our purposes mass and weight are the same quantity.
density
Also called specific weight, density is mass per unit volume, and may be registered as lb./cu.ft., lb./gal., grams/ml., grams/cu.meter. Taking a fixed volume container, filling it with a fluid, and weighing it, we can determine density of the fluid (after subtracting the weight of the container).
One cubic foot of water weighs 62.4 pounds. (Density = 62.4 lb./cu.ft.)
One gallon of water weighs 8.34 pounds. (Density = 8.34 lb./gal.)
One milliliter of water weighs 1 gram. (Density = 1 gram/ml.)
temperature
The density of materials is affected by temperature. Many solids expand when hot. Fluids also become less dense when warm. Water has the peculiar property of being most dense at four degrees Celsius. Above and below that temperature, it expands. This accounts for seasonal turnover in lakes, and the formation of density gradients, or layers, in reservoirs and behind dams, often concentrating pollution at specific depths. In settling tanks and clarifiers, this effect can inhibit uniform mixing of influent water, causing short circuiting. Most solids will settle more effectively in warm waters than in cold waters because the water is denser when cold.
pressure
Though the density of gases is greatly affected by pressure, the density of most liquids, including water, is not. For all practical purposes, water is considered an incompressible fluid; 62.4 pounds of water occupies a volume of one cubic foot, regardless of the pressure applied to it.
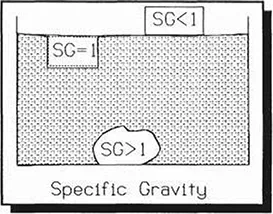
specific gravity
This value designates the ratio of the density of a substance, compared to the density of a standard substance. It is a way to specify relative densities. For liquids and solids, the standard chosen is the weight of water. Therefore:
If the Specific Gravity of water were to be determined this way, we would write:
The Specific Gravity of water is 1.
Substances with a Specific Gravity of less than 1 will float (gasoline, styrofoam, wood, wastewater scum). Substances with a Specific Gravity of more than 1 will sink (bricks, steel, grit, floe, sludge). A good example of treatment process taking advantage of different Specific Gravities is the multi-media filter. During backwash, the different grades of media are mixed up, but at the end of the backwash cycle, as the water quiets, the media layers become separate and distinct: anthracite on top, sand in the middle, garnet on the bottom. These substances are chosen for filtration partially because of their different specific gravities.
An anaerobic digester is another good example of the effect of Specific Gravity. Separation of solids, liquid and gas is essential to operation, enabling each to be drawn off separately.
Just as the density of liquids and solids are compared to water as the standard, the Specific Gravity of gases is based on the density of air. Air weighs .075 pounds/cubic foot at standard temperature and pressure. Specific Gravity calculations are done in the same manner, and will determine whether a gas rises or falls in a room full of air.
Once Specific Gravity is known, density and weight can be calculated. Note that Specific Gravity is a pure number with no unit label. The units have cancelled out in the calculation.
displacement
A solid submerged in a body of water will displace its own volume. The displacement causes a rise in water level equivalent to the volume of the submerged object. An object partially submerged displaces a volume of water equivalent to the submerged section of the object.
Sometimes it is useful to know the volume of an irregularly shaped object. This can be determined by measuring the displacement. For example, to calculate the percent volume of mudballs in a sand filter, take a core sample with a small cylinder of known volume. Extracting a portion of media, sift out the sand, leaving only the mudballs. Pour them into a large graduated cylinder, filled to a liter with water. A volume of water will be displaced in the cylinder equivalent to the volume of the mudballs. Record the milliliter rise in the cylinder, divide by the volume of the core sampler, and you have the percent mudballs - in the sampler, and in the sand filter.
buoyancy
Buoyancy is known as Archimedes’ Principle, and has been employed by man for over two thousand years. An object submerged in a liquid is subject to an upward force equal to the weight of the volume of liquid it has displaced. A dramatic example of this is the underwater swimmer, whose body is lighter than the weight of the water that he is displacing. He actually assumes a negative weight underwater, which causes him to fall upward, unless he purposely swims down. This force of buoyancy applies to any submerged object. For example, a cubic foot of steel weighs 486.7 pounds in air. But immersed in water it weighs 486.7 lb. - minus the weight of the cubic foot of water it has displaced (the force of buoyancy).
Problems
- The water in a tank weighs 820 pounds. How many gallons does it hold?
- If the municipal water rate is one dollar per thousand gallons, how many pounds of water are delivered to the customer’s house - for a dollar?
- Three cubic feet of gasoline weighs 131 lb.
- What is the Specific Gravity of gasoline?
- How much does a gallon of it weigh?
- If an oil weighs 55 lb./cu.ft., how much does a five gallon can of it weigh?
- The Specific Gravity of a liquid chemical is 1.27. What is its density (lb./cu.ft.)?
- A truck is designed to transport 5000 gallons of liquid.
- How many pounds of water can it transport?
- How many pounds of sulfuric acid (SG= 1.83)?
- What is the Specific Gravity of concrete if it weighs 150 lb./cu.ft.?
- The Specific Gravity of mercury is 13.6.
- What is its weight per cubic foot?
- Per cubic yard?
- If the density of a sand is 100 lb/cu.ft., how much does a cubic yard of this sand weigh?
- Chlorine gas is 2.5 times heavier than air.
- What is the weight of a cubic foot of chlorine?
- What would a room (10’ x 12’ x 8’) full of chlorine gas weigh?
- The operator is pumping a solution of alum (density = 83 lb./cu.ft.) at a rate of 3 gpm into a chemical treatment system.
- How many pounds are fed in one day?
- What is the Specific Gravity of the alum solution?
- A digested sludge whose specific gravity is 1.25 is pumped at 50 gpm to the drying beds.
- How many pounds of digested sludge are applied to a bed which is 30 ft. wide, 60 ft. long, and 10 inches deep?
- How long does it take to fill this bed?
- A solid piece of plastic whose specific gravity is 1.2 is dropped from a boat into a lake. How deep will it sink?
- A gallon of pure mercury is poured into a 24 inch diameter pail. If the mercury weighs 113.4 lb., what is the depth of the mercury in the pail?
- A wastewater grit chamber measures 5 ft. wide, 30 ft. long, and has a 24 inch water depth. If 22,000 lb. of grit (Specific Gravity = 2.2) were to accumulate in the bottom of this chamber, what would be the new water depth?
- A stone weighs 90 lb. in air. When immersed in water, it weighs 50 lb.
- What is the volume of the stone?
- What is its Specific Gravity?
- If a 150 lb. person swims in a pool, how many cubic feet of water will he displace if his Specific Gravity is 1.1?
- A heat exchanger is being constructed to maintain temperature of the anaerobic digester. Sludge will pass through a coiled 4 inch diameter pipe 160 ft. long, which is set inside a hot water tank 4 ft. by 4 ft. by 4 ft. high. How many gallons of water will be needed to fill this tank?
- An open box is to be sunk to its rim in water. If its dimensions are 10 ft. by 10 ft. by 8 ft. deep, how many pounds must it weigh in order to stay submerged?
- A cubic block of concrete 4 ft. on a side is submerged in a tank of water which measures...
Table of contents
- Cover
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Introduction
- Chapter 1 Mass, Density and Displacement
- Chapter 2 Flow and Velocity
- Chapter 3 Pressure
- Chapter 4 Bernoulli’s Theorem
- Chapter 5 Pumping - Introduction
- Chapter 6 Friction Loss
- Chapter 7 Compound Pipes
- Chapter 8 Minor Head Loss
- Chapter 9 Open Channel Flow
- Chapter 10 Flow Measurement I Flow Rate Meters
- Chapter 11 Flow Measurement II Totalizer Meters
- Chapter 12 Centrifugal Pumps I
- Chapter 13 Centrifugal Pumps II
- Chapter 14 Positive Displacement Pumps
- Appendix I Problem Solutions
- Appendix II Conversions
- Appendix III Important Formulas
- Appendix IV Formula Derivations
- Appendix V Numeric Tables
- Appendix VI Pump Troubleshooting
- Glossary
- Sources Consulted
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Practical Hydraulics Handbook by Barbara Hauser in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Environmental Science. We have over one million books available in our catalogue for you to explore.