
eBook - ePub
Schizophrenia
The Final Frontier - A Festschrift for Robin M. Murray
This is a test
- 400 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Schizophrenia
The Final Frontier - A Festschrift for Robin M. Murray
Book details
Book preview
Table of contents
Citations
About This Book
Schizophrenia is a unique project reflecting the contribution that Robin M. Murray has made to the field of psychiatry over the past 35 years, with a particular focus on the advances that have been made to the understanding and treatment of schizophrenia.
International contributors have been brought together to pay tribute to Robin Murray's work and explore the latest findings in the area. Sections cover:
- neurodevelopment
- neuroscience and pharmacology
- neuroimaging
- genetics
- cognition
- social psychiatry
- treatment.
-
This book will be essential reading for psychiatrists, clinical psychologists, social and basic scientists whose work is related to major mental illness, as well as admirers of the work of Robin Murray.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Schizophrenia by Anthony S. David, Shitij Kapur, Peter McGuffin, Anthony S. David, Shitij Kapur, Peter McGuffin in PDF and/or ePUB format, as well as other popular books in Psychologie & Klinische Psychologie. We have over one million books available in our catalogue for you to explore.
Information
Part I
Development
1 The Neurodevelopmental Hypothesis of Schizophrenia
Schizophrenia has an insidious onset, with full manifestation of the disorder occurring in late adolescence and early adulthood. Onset is complex and includes cognitive and social changes in early childhood as well as a more proximal prodromal phase in which functioning undergoes a pronounced deterioration and subpsychotic symptoms emerge. Based on this trend of slow change, it was initially thought that schizophrenia might be degenerative, similar to other gradually emerging disorders such as Alzheimer’s disease. However, with an increased understanding of the associated neural changes, a neurodegenerative process appeared less likely. For instance, despite early findings (Weinberger, Wagner, & Wyatt, 1983), it became evident that any gliosis present in schizophrenia could not explain the degree of neuroanatomical changes observed (Harrison, 1999). Further, findings that ventricular enlargement (originally presumed to be evidence of neural degeneration) positively correlated with a history of obstetric complications (OCs; Reveley, Reveley, & Murray, 1984) supported the importance of environmental influences on early development. Simultaneously, a deeper understanding of later neurodevelopmental processes began to emerge, notably that normal development includes a natural pruning process that can in itself cause a decrease in cortical thickness or brain volume (Huttenlocher, 1979) and that in areas critical for schizophrenia, such as the prefrontal cortex, these processes reach completion around the age of disease onset (Huttenlocher, 1990; Pfefferbaum et al., 1994). The confluence of these findings, with work by Robin Murray at the forefront, lead to the emergence of the widely influential neurodevelopmental hypothesis of schizophrenia, in which a subtle, static brain lesion (caused by a combination of genetic and environmental factors) later interacts with normal maturational processes in the brain to result in schizophrenia (Murray & Lewis, 1987).
Ongoing work unraveling the neurodevelopmental hypothesis indicates that there are three key periods across the lifespan that may influence risk for and development of schizophrenia: (1) conception; (2) early developmental; and (3) later developmental periods (see Figure 1.1). First, at conception, the genetic make-up is determined, and genetic liability for the disorder, in the form of risk genes, is conferred. While genetic liability is instantiated at conception, the degree of risk is not fixed; rather, there can be interactive effects leading to changes in risk across the lifespan even against the same genetic background. For instance, predisposing genotypes may interact with both normal and disrupted developmental processes and environmental events that occur during specific phases of development. Two primary periods are likely to have significant influence. The “early” period includes pre- and perinatal brain development and may be impacted by factors such as OCs (Lewis, Owen, & Murray, 1989) and prenatal maternal infection (Buka, Cannon, Torrey, & Yolken, 2008). Further, these factors may interact with genetic liability for the disorder to result in neuroanatomical changes seen in the disorder (T. Cannon, van Erp et al., 2002; van Erp et al., 2002). These pre- and perinatal disturbances may interfere with some fundamental process during the development of neural circuitry, thereby leaving the organism more vulnerable to potential insults later in development. The presence of a very early developmental change is consistent with the view that the neural changes associated with the development of schizophrenia are partly present from birth and may therefore explain early neurocognitive and social deficits in many of the children who later develop schizophrenia (Bearden et al., 2000; Cannon, Huttunen et al., 2002; T. Cannon et al., 2000; Jones, Rodgers, Murray, & Marmot, 1994; Rosso et al., 2000; St Clair et al., 1990).
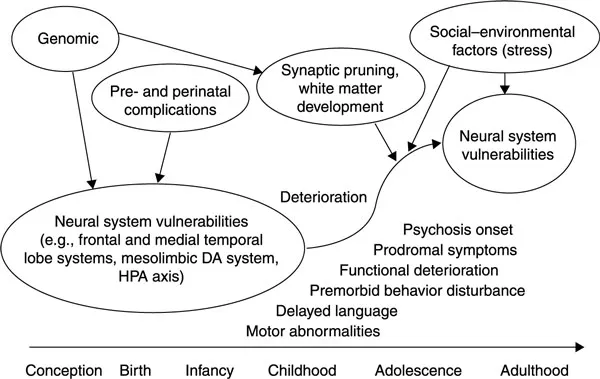
Figure 1.1 Developmental model of schizophrenia.
However, despite these early influences, and the presence of observable differences in childhood, formal diagnostic symptoms and signs of schizophrenia still do not fully emerge until late adolescence or early adulthood. Thus, risk factors occurring during this “later” developmental period may also play an important role in disease onset. Late adolescence and early adulthood represents a period of continuing developmental brain change and refinement, as well as a period of substantial neuroendocrine influence. Importantly, a normal pruning process takes place (Feinberg, 1982; McGlashan & Hoffman, 2000) reducing extraneous excitatory synapses and honing more mature circuitry. Findings of progressive anatomical deterioration in schizophrenia (Gur et al., 1998; Thompson et al., 2001) suggest that additional factors active during the late developmental period and more proximal to the onset of schizophrenia, in addition to early acting genetic and perinatal risk factors, may play a role in the etiology and pathophysiology of schizophrenia. Here we discuss the relationship of recent findings in these early and late periods, including those from our own research group, as related to the neurodevelopmental model and to Dr Murray’s work.
Early Developmental Changes
Support for the involvement of early stage developmental factors comes from repeated associations between OCs and risk for schizophrenia (Cannon, Jones, & Murray, 2002) and findings that schizophrenia patients are more likely to have OCs than other psychiatric patients (Lewis & Murray, 1987). OCs encompass any aberration in normal development occurring in the prenatal, perinatal, and early neonatal periods (McNeil, 1988). A number of OCs have been found in the histories of patients with schizophrenia, including low birth weight, exposure to maternal infections during pregnancy, fetal hypoxia (lack of oxygen to the fetus), malnutrition during pregnancy, and maternal diabetes during pregnancy (Brown, 2006; Cannon, Jones et al., 2002; T. Cannon, 1997; Dalman et al., 2001; Ichiki et al., 2000; Susser, St Clair, & He, 2008). One type of OC, fetal hypoxia, has been found in 20–30% of patients with schizophrenia, a rate substantially higher than that in the general population, 5–10% (Buka, Tsuang, & Lipsitt, 1993).
These studies support the notion that exposure to certain environmental conditions could alter the course of early development resulting in schizophrenia. Nevertheless, the question remains whether OCs cause schizophrenia on their own (phenocopy model), depend on genetic influences (interaction model), aggregate with genetic influences (additive influences), are correlated with genetic influences (gene–environment covariation), and/or change the function and structure of genes (epigenetic influences). Our research group, and others, have addressed these questions in order to determine the relative contributions of genetic and obstetric factors to the neurodevelopmental course of schizophrenia (Mittal, Ellman, & Cannon, 2008).
Determining the correct model is critical for understanding whether OCs contribute to the developmental course of schizophrenia and has implications for primary prevention and early intervention strategies. If the gene–environment covariation model is correct, OCs may be correlated with the genes for schizophrenia and obstetric events would be confounded with genetic factors. Studies finding an increased prevalence of OCs in the pregnancies and deliveries of mothers diagnosed with schizophrenia support this model (Bennedsen, 1998). Although mothers with schizophrenia clearly carry risk-genes, they also show increased health-risk behaviors during pregnancy that are themselves associated with OCs (Delpisheh, Kelly, Rizwan, & Brabin, 2006; Raatikainen, Huurinainen, & Heinonen, 2007; Smith et al., 2006), such as lack of prenatal care, polydrug use, alcohol consumption, use of psychiatric medications, and smoking (Bennedsen, 1998). Moreover, discontinuation of antipsychotic medication during pregnancy has been associated with worsened symptoms, which impact prenatal health via factors such as increased stress, poor nutrition, poor self-care, suicide attempts, attempts at premature delivery, and other deleterious behaviors (McNeil, Kaij, & Malmquist-Larsson, 1984; Miller, 1997).
To test whether increases in OCs are associated with risk-genes and/or increased health-risk behaviors, our group has conducted population-based studies in Finland. First, we found that mothers with a genetic liability for schizophrenia (a first-degree relative with schizophrenia) exhibit no increased risk of OCs compared to control mothers (Ellman, Huttunen, Lönnqvist, & Cannon, 2007). This finding substantially weakens the gene–environment covariation model, which would predict increased OCs among women with genetic risk. Second, we found that smoking mediated the relationship between maternal schizophrenia status and decreased birth weight, suggesting that health-risk behaviors may, in part, account for the increased prevalence of OCs in offspring of mothers with schizophrenia (Ellman et al., 2007). Although we observed a higher rate of OCs in the pregnancies and deliveries of mothers diagnosed with schizophrenia, we only examined one health-risk behavior in this study, therefore a number of other experiential factors associated with schizophrenia could potentially account for this observed result.
OCs may also result in a more severe form of schizophrenia characterized by an earlier age of onset, more severe premorbid difficulties, and more pronounced alterations in brain structure. Associations have been found with OCs and earlier onset (Verdoux et al., 1997). Accordingly, we have found that hypoxia-associated OCs were significantly related to increased risk of schizophrenia, which appeared to be restricted to cases with an earlier age of onset (T. Cannon, van Erp et al., 2002; Rosso et al., 2000). In addition, a prospective study following high-risk (i.e., prodromal) adolescents found that a history of OCs (including hypoxia-associated events) significantly increased the odds of conversion to an Axis-I psychotic disorder in a two-year period and was strongly associated with both positive and negative symptomatology (Mittal et al., 2009). This is consistent with the idea that early insults may interact with processes that occur throughout development to influence timing of disease onset. Further, our data indicate that increases in hypoxia-associated OCs were not observed in the histories of unaffected siblings of schizophrenia cases, diminishing the possibility of gene–environment covariation with respect to this class of OCs.
Exposure to OCs also results in childhood motor and cognitive problems that appear to be restricted to children who later develop schizophrenia. Specifically, hypoxia-associated OCs predicted unusual movements at age 4, but not age 7, among children who developed schizophrenia but not in unaffected siblings or controls (Rosso et al., 2000). Similarly, we have found that serologically documented maternal influenza infection during pregnancy was related to decreases in verbal IQ scores among 7-year-old offspring who developed schizophrenia in adulthood (Ellman, Yolken, Buka, Torrey, & Cannon, 2009) and not among control children with similar exposure. These findings suggest that a genetic and/or environmental factor associated with schizophrenia likely rendered the fetal brain vulnerable to the effects of fetal hypoxia and infection, resulting in childhood difficulties occurring far before the onset of psychotic symptoms.
In addition, it has been found that a history of hypoxia-associated OCs is associated with more pronounced brain abnormalities in patients. While seminal work by Robin Murray’s group first indicated that in patients with schizophrenia, OCs such as intraventricular hemorrhage were correlated with ventricular enlargement in adulthood (Murray, Lewis, &Reveley, 1985;Reveley etal.. 1984), our group has extended this to find that hypoxia-associated OCs predicted ventricular enlargement in patients, but not their unaffected siblings or controls (T. Cannon, van Erp et al., 2002). Similarly, hippocampal volume decreases with genetic liability for schizophrenia, with the smallest volumes among patients, followed by their unaffected siblings, and then control participants. Among the cases with schizophrenia, a history of hypoxia-associated OCs was associated with further reductions in hippocampal volumes, suggesting that genetic contributions to hippocampal volume reductions in patients were worsened by a history of fetal hypoxia (van Erp et al., 2002). These data suggest that brain anomalies commonly found among patients with schizophrenia (Wright et al., 2000) may have some neurodevelopmental origins. Moreover, our findings further support the possibility that there is some existing vulnerability for schizophrenia that renders the fetus susceptible to the damaging influences of OCs, such as fetal hypoxia and maternal infection.
Further support for this possibility comes from recent work examining brain derived neurotrophic factor (BDNF) levels in umbilical cord sera from those who developed schizophrenia in adulthood compared to non-psychiatric controls (T. Cannon, Yolken, Buka, & Torrey, 2008). BDNF in cord sera is entirely from fetal origins and plays a critical role in neuronal development, as well as the survival of neurons under stressful conditions (T. Cannon et al., 2008; Lee et al., 2004; Meng et al., 2005). Among control sera, hypoxia-associated OCs were associated with an increase in BDNF levels; this was expected given that fetal hypoxia should evoke a neuroprotective BDNF response by the fetus. In contrast, among cases, hypoxia was associated with decreased BDNF that was not explained by other OCs or by the BDNF Val66Met polymorphism. These findings suggest that there is disrupted neurotrophic signaling in response to hypoxia-associated pre- and perinatal complications among those who ultimately develop schizophrenia.
Cumulatively, the evidence on pre- and perinatal complications supports the neurodevelopmental hypothesis and suggests that these factors may lead to a more severe form of schizophrenia. Further, there is preliminary evidence for the possibility that genetic susceptibility to schizophrenia renders the fetus vulnerable to the influences of specific OCs. However, it remains unclear whether obstetric events lead to a casc...
Table of contents
- Cover Page
- Half Title Page
- The Maudsley Series
- Title Page
- Copyright Page
- Contents
- List of plates
- List of figures
- List of tables
- List of contributors
- Foreword by Daniel R. Weinberger
- Preface
- Acknowledgements
- Part I Development
- Part II Neuroscience
- Part III Neuroimaging
- Part IV Genetics
- Part V Cognition
- Part VI Social Psychiatry
- Part VII Treatment
- Part VIII Afterword
- Index