
Genetic Engineering Fundamentals
An Introduction to Principles and Applications
- 304 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About This Book
This important reference/text provides technologists with the basic informationnecessary to interact scientifically with molecular biologists and get involved in scalinguplaboratory procedures and designing and constructing commercial plants.Requiring no previous training or experience in biology, Genetic EngineeringFundamentals explains the biological and chemical principles of recombinant DNAtechnology... emphasizes techniques used to isolate and clone specific genes frombacteria, plants, and animals, and methods of scaling-up the formation of the geneproduct for commercial applications... analyzes problems encountered in scaling-upthe microprocessing of biochemical procedures... includes an extensive glossary andnumerous illustrations... identifies other resource materials in the field... and more.Presenting the fundamentals of biochemistry and molecular biology to workers andstudents in other fields, this state-of-the-art reference/text is essentiai reading fortechnologists in chemistry and engineering; biomedical, chemical, electrical andelectronics, industrial, mechanical, manufacturing, design, plant, control, civil, genetic, and environmental engineers; chemists, botanists, and zoologists; and advancedundergraduate and graduate courses in engineering, biotechnology, and industrialmicrobiology.
Frequently asked questions
Information
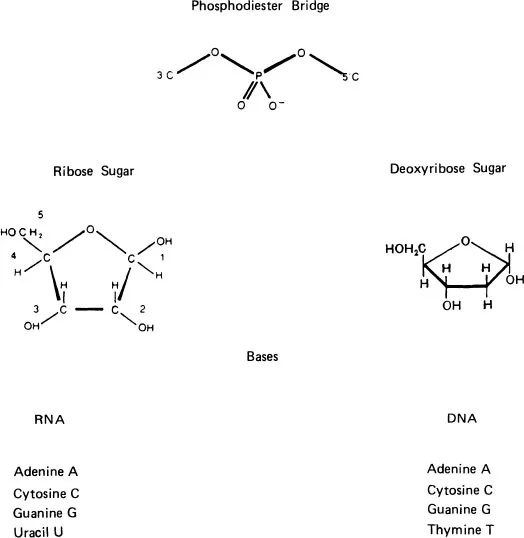
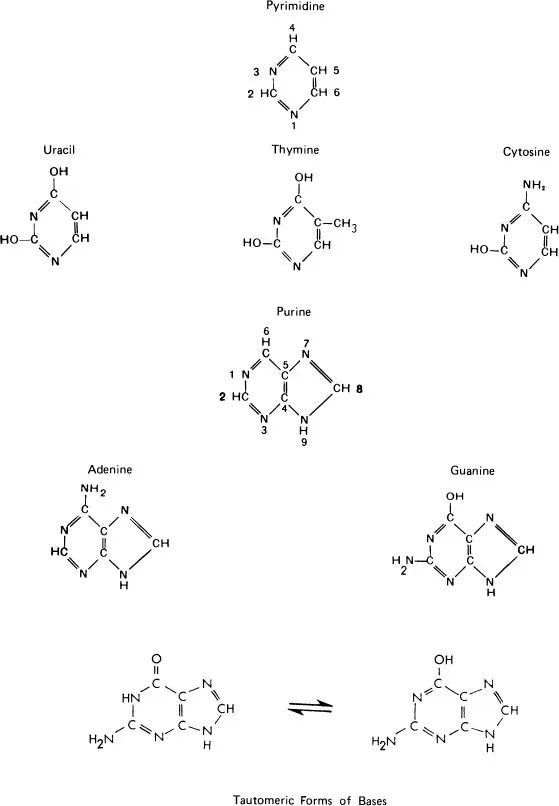
Base | Ribonucleoside | Ribonucleotide (5’-monophosphate) |
Adenine (A) | Adenosine | Adenylate (AMP) |
Guanine (G) | Guanosine | Guanylate (GMP) |
Uracil (U) | Uridine | Uridylate (UMP) |
Cytosine (C) | Cytidine | Cytidylate (CMP) |
Deoxyribonucleoside | Deoxyribonucleotide (5’-monophosphate) | |
Deoxyadenine (A) | Deoxyadenosine | Deoxyadenylate (dAMP) |
Deoxyguanine (G) | Deoxyguanosine | Deoxyganylate (dGMP) |
Deoxythymidine or Thymine (T) | Deoxythimidine or Thymidine | Deoxythymidylate or Thymidylate (dTMP) |
Deoxycytosine (C) | Deoxycytidine | Deoxycytidylate (dCMP) |
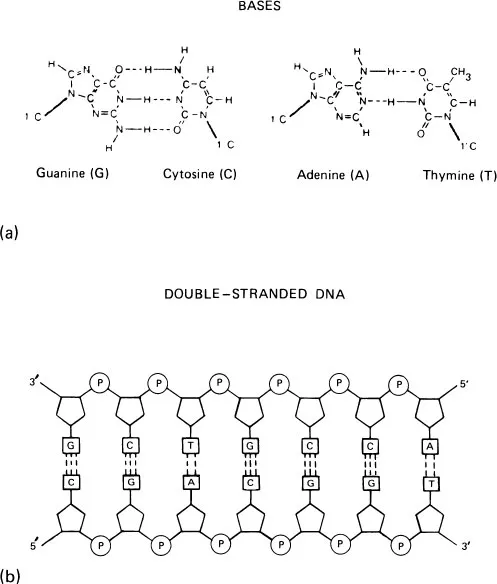
DNA | RNA |
Deoxyribose | Ribose |
Thymine | Uracil |
(double-stranded) | (usually single-stranded) |
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Introduction
- 1. Basic Components
- 2. The Cell
- 3. DNA, RNA, and Genes
- 4. Protein Synthesis
- 5. Enzymes
- 6. Plasmids, Viruses, and Microbial Hosts–Vectors
- 7. Recombinant Techniques
- 8. Nucleotide Sequencing and Hybridizing
- 9. Plant Genetics
- 10. Genetic Engineering Activities
- 11. Technology and Design
- 12. Techniques with Mammalian Cells
- 13. Precautions and Regulations
- 14. Update
- Appendices
- Index