
eBook - ePub
Reactor Design for Chemical Engineers
- 454 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Reactor Design for Chemical Engineers
About this book
Intended primarily for undergraduate chemical-engineering students, this book also includes material which bridges the gap between undergraduate and graduate requirements. The introduction contains a listing of the principal types of reactors employed in the chemical industry, with diagrams and examples of their use. There is then a brief exploration of the concepts employed in later sections for modelling and sizing reactors, followed by basic information on stoichiometry and thermodynamics, and the kinetics of homogeneous and catalyzed reactions. Subsequent chapters are devoted to reactor sizing and modelling in some simple situations, and more detailed coverage of the design and operation of the principal reactor types.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 Introduction
1.1 The need for good reactor design
In recent years, the chemical reactor has been elevated to the position of being arguably the most important unit to optimise within a chemical processing plant. Until the early 1970s, downstream separation processes were regarded as the key to profitable operation of a chemical plant. While every effort was made to obtain both high selectivity and conversion to the desired product within the reactor, the desired product purity was achieved largely by well-designed downstream separation operations.
The increased cost and less certain availability of feedstocks, along with legislation to protect the environment, has now led to a drive towards ‘clean technology’, with the objectives of more efficient processing and waste minimisation. The latter aspect is not only economically important but also contributes to a determined effort to ensure that harmful material is not present in effluent streams leaving chemical plant. The above objectives are better achieved by improvements in reactor technology to reduce the amount of waste material produced than by the installation of retrofit downstream units to remove low-value by-products and other waste from effluent streams, although, as a last resort, this may also be necessary.
The new objectives are centred around clean technology synthesis and are namely: (i) to give as far as possible only the desired product; (ii) to reduce by-products and pollution to zero or very small proportions; (iii) to intensify processing to give improved economics; and (iv) to ensure that the process products are used in an environmentally friendly manner with emphasis on recycling and re-use.
Progress has been achieved towards meeting these objectives and this has led in recent years to increased interest in the reactor and reaction chemistry in comparison with downstream processing. Two factors which have led to this have been (i) improvement in reactor design combined with the availability of precise kinetic data and powerful modelling and computational techniques and (ii) the design and availability of novel and highly selective catalysts. Catalyst design, and some achievements in this area, is discussed in Chapter 6. In the modern chemical industry the majority of processes depend upon the use of catalysts and it is likely that an even higher proportion of new processes will do so.
A review of the principal reactor types currently in use follows: more quantitative discussions of the design and characteristics of many of these will be found in Chapter 4 and subsequent chapters.
1.2 Reactor types
There are three main types of chemical reactor namely:
(a) batch
(b) continuous
(c) semi-batch or semi-continuous.
These general reactor types can be divided into a number of subcategories according to the nature and manner of processing the feedstocks, as follows:
(a) single phase: gas, liquid or solid
(b) multi-phase, which can be described overall as fluid–solid but may encompass combinations of:
(i) gas–liquid
(ii) liquid–liquid
(iii) gas–solid (catalysed or uncatalysed)
(iv) liquid–solid (catalysed or uncatalysed)
(v) gas–liquid–solid (catalysed or uncatalysed).
A large number of reactions involving solids are catalytic. In such cases, the catalytic solid is not consumed by the reaction and, ideally, can be reused for a considerable period of time.
Batch and semi-batch reactors operate in various degrees of unsteady state. Batch reactors are relatively easy to describe since the variable and unsteady operating parameter is the concentration and all material is contained within the reactor during operations. Simple kinetic equations can therefore be used to describe the course of reaction. In the case of semi-batch reactors, however, some materials which are charged to the reactor remain there for the course of the reaction, but others may be added and/or removed continuously.
Continuous reactors, as their name implies, operate under steady-state conditions, feedstock and product being added and removed respectively at a steady rate. The only exception to this is at start-up and shut-down of the reactor.
Reactors operated continuously can be represented by two extremes of flow regime. The first of these is the ‘plug flow’ reactor (PFR) for which there is no mixing of fluid elements as they pass through the reactor. In this case there is a continuous concentration gradient for the various species from inlet to outlet. The other extreme is that represented by the ‘perfectly mixed’ reactor (PMR). In this case, the reactor contents are so well mixed that there is no concentration gradient within the reactor and the effluent concentration is identical to the concentration within the tank. In practice, by careful design both ideal flow patterns can be closely approached. A very close approximation to ‘plug flow’ is achieved in many of the tubular reactors used in the heavy chemicals industry while a close approximation to ‘perfect mixing’ is achieved in the continuous stirred tank reactors (CSTRs) described in Section 1.4.1.1. In both the above cases, approach to ideal behaviour can be sufficiently close to be useful for design purposes. Of course, some reactors exhibit flow regimes that are intermediate between the two ideals of the PFR and PMR. These reactors can be described as non-ideal, and the effects of non-ideal flow on conversion will be discussed in Chapter 5.
1.3 Batch reactors
Batch reactors process one batch of material at a time in a closed system. Typical types are shown in Figure 1.1. They have the advantage of flexibility in that they can be used to produce a variety of chemicals, i.e. they are not dedicated to a single product. They are used for fine chemical, pharmaceutical and polymer production on a relatively small scale. An additional advantage of batch reactor is that of relatively small capital investment. Nevertheless, their design and operation are being improved to meet the needs of clean technology operations, and the Buss reactor (shown later in Figure 1.5) is one example where improvements in batch reactor technology have led to significantly enhanced performance. The important operational parameters in the case of batch reactors are good mixing and heat transfer. The need for this is well illustrated by considering the case of a gas–liquid–solid catalysed exothermic reaction. Good mixing is particularly important to give a homogeneous dispersion of all species and to ensure good mass and heat transfer between the phases. Provision of good external heat exchange may also be necessary. Various batch reactor designs can be seen in Figure 1.1(a)–(d).
The most appropriate design for the stirrers in these reactors depends on the nature of the fluid phases requiring mixing. Figures 1.2 and 1.3 show typical turbine-type and wide-radius agitators, while Figure 1.4 illustrates several ‘Archimedes’ screw’ and marine propeller-type agitators. Figure 1.4 also shows the types of circulation obtained from these and other stirring systems, and in a number of situations it is necessary to use baffles to control vortex formation. Turbine-type agitators can be used in liquids with viscosity up to about 10 Pa s[1]. Wide-radius or spiral agitators are required for the very viscous liquids with viscosities greater than this.
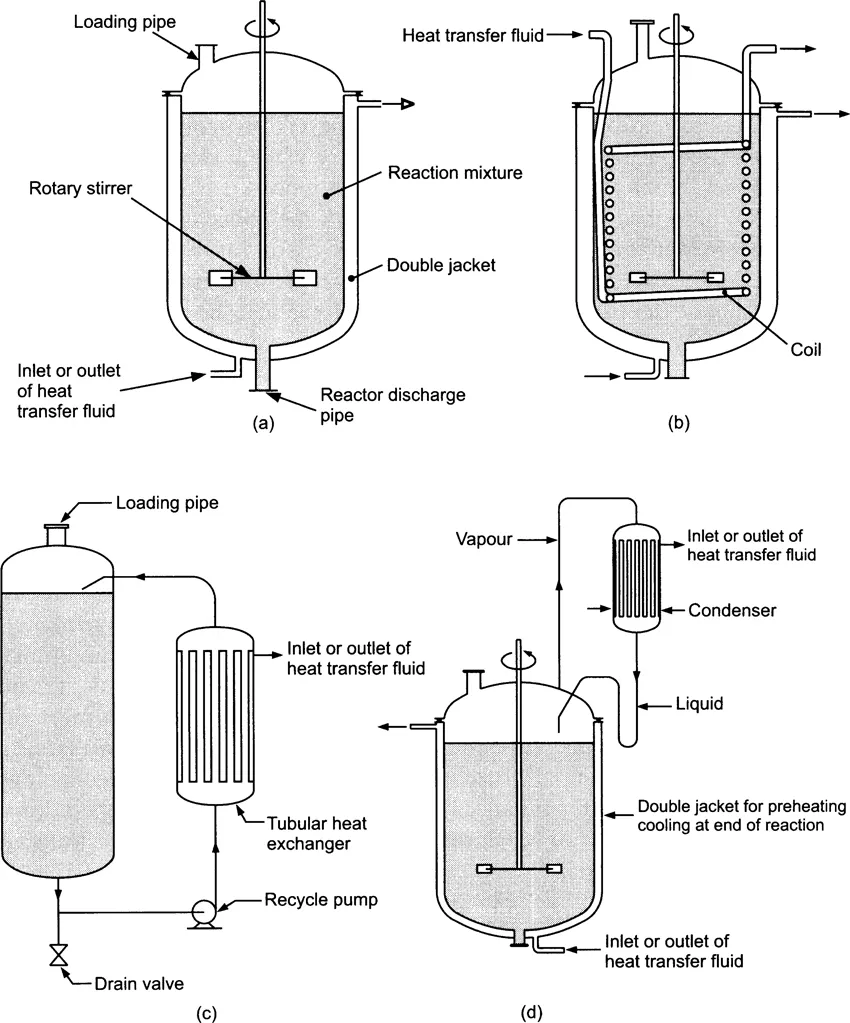
Figure 1.1 Some typical types of batch reactor: (a) batch reactor with double jacket; (b) batch reactor with double jacket and internal coil; (c) batch reactor with external heat exchanger on circulation loop; (d) batch reactor with cooling by vapour phase condensation and recycle. (From Trambouze et al. (1988), p. 98; reprinted by permission of Gulf Publishing Co., Editions Technip, 27 Rue Ginoux, Paris.)
Another method of inducing good mixing and heat transfer involves the use of closed loop circulation. Figure 1.1(c) shows a simple closed loop circulation reactor while Figure 1.5(a) and 1.5(b) show highly efficient three-phase reactors, the former being the Buss reactor [2] and the latter the Cocurrent Downflow Contactor Reactor (CDCR) [3]. These reactors are similar in many ways, the Buss reactor using a venturi device to mix gas with liquid and solid, while the CDCR employs a si...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Contributors
- Preface
- 1 Introduction
- 2 Reaction stoichiometry and thermodynamics
- 3 Kinetics of homogeneous reactions and of reactions on solid catalyst surfaces
- 4 Simple reactor sizing calculations
- 5 Non-ideal flow in chemical reactors and the residence time distribution
- 6 Catalyst design and manufacture
- 7 Overview of catalytic reactor design
- 8 Fluidised bed reactors
- 9 Three-phase reactors
- 10 Bioreactors
- Symbols
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Reactor Design for Chemical Engineers by J. M. Winterbottom, Michael King, J. M. Winterbottom,Michael King in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.