1.1 The ubiquitous nature of electrochemistry
The subject of electrochemistry is concerned with the study and exploitation of the transference of electrical charges across interfaces and through solution. Transfer of material across biological membranes, storage of electricity in batteries, the production of nylon, electroplating, nerve action, corrosion—these are some areas in which few people would argue that the phenomena are electrochemical.
Yet within the more academic tradition, electrochemistry has been seen as a large and important area of physical chemistry—in many respects rightly so. The subject finds its place in courses of physical chemistry and it appears in chapters of books of that subdivision of the subject of chemistry. Still, it is difficult to define the limits of electrochemistry, for its influence permeates so much of wider chemistry.
The Periodic Table of the Elements, the foundation of systematic inorganic chemistry, is complemented by the Electrochemical Series. Any discussion of periodicity quickly introduces such terms as electronegativity and ionic character—and the language has turned inexorably to that of electrochemistry. Metal extraction and chemical analysis require electrochemical principles for their understanding and effective exploitation, both on an industrial and on a laboratory scale. Organic synthesis increasingly sees electrochemistry put to use; modern development owes much to the control of electrochemical parameters made possible by modern instrumentation although the Kolbe synthesis was established in the mid-nineteenth century.
1.3 The domains of electrochemistry
Electrochemistry is concerned with charges and with their movement and transfer from one medium to another. The ultimate unit of charge is that carried by the electron; electrons are important in electrochemistry and their functions here are similar to some of those which they exhibit in related disciplines more usually regarded as the province of physics. The science of Thermionics is built on the exchange of electrons between a solid and a vacuum; that of transistor electronics is based on their transfer between one solid phase and another.
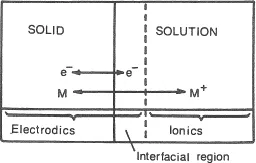
When electrons exchange between metals (or other electronic conductors), in this context usually termed electrodes, and species in solution within which that metal is placed, Electrodics is a suitable name to give to the discipline which emerges.
The behaviour of species in solution with an excess or deficiency of electrons, so that they form the negatively or positively charged entities termed ions, is the interest of the science which may be called Ionics. Electrodics, concerned with electrode processes, and ionics, concerned with the behaviour of ions in solution, constitute much of the fabric ofelectrochemistry.
Separating the solid (or sometimes liquid) material of an electrode and a solution there exists an interfacial region of great importance. The interplay of these three regions is implied by Figure 1.1.
The extremely large field gradients at electrode/solution interfaces caused by imposed potential differences, induce gross distortions in the positions (orbitals) which electrons may occupy in solute ions and in electrodes. Such constraints affect the transfer of electrons between the solid and solution species.
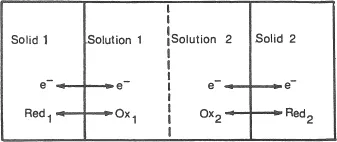
Systems of the sort shown in Figure 1.1 only take on practical significance when combined in pairs to form electrochemical cells (Figure 1.2). Differing chemical characteristics of systems labelled 1 and 2 give rise to voltages across terminals connected to the electrodes. This is the arrangement in the wide range of batteries commercially available, except that pastes often replace solutions, render the arrangement stationary and lead to the description dry. In cases where the processes which generate the cell voltage can be reversed, after discharge, by imposition of an external voltage, the battery is rechargeable. The efficiency of such processes is largely governed by the absence of side reactions, the lead-acid cell installed in our motor cars being particularly effective. In other variations, a voltage is generated by the transfer of electrons between the electrodes and fuels continuously supplied to the adjacent solution sectors. This is easier said than done and it is necessary to have appropriate catalysts incorporated to ensure rapid electron transfer. Manned spacecraft use hydrogen and oxygen in such fuel cells to provide a major part of the electricity requirements, and to supplement the water supply by this product of the process.
Figure 1.1 Electrodics is concerned with the exchange of electrons between electrodes and species in solution (these are frequently ions). Ionics is the discipline concerned with the behaviour of ions in solution.
Figure 1.2 General arrangement of an electrochemical cell. Spontaneous generation of a voltage occurs by interfacing of certain electrode/solution pairs of differing chemical characteristics. Imposition of external voltage can reverse the discharge process in rechargeable cells. Supply of ‘fuels’ to solution sectors under conditions that electron exchange may occur between them and the electrodes can generate an exploitable voltage.
The arrangement shown in Figure 1.2 is quite general and of particular practical importance is the variant where one half-cell has its properties maintained constant so that relative changes in the other half may be investigated. This approach is the basis of much of electroanalytical chemistry.
The nature, behaviour and structure of species in solution are closely linked with the way in which they undergo electron exchange reactions. Changing the form of these species, such as by complexation, often produces a change in electrode behaviour. The nature of metallic deposits produced in electroplating is, for example, significantly affected by the presence of additives to the solution. Even today the reasons for this are far from completely understood and the production of many plated surfaces remains as much an art as a science.
In many arrangements, the magnitudes of currents and voltages are related to the concentrations of dissolved solutes. Measurement of such quantities, under carefully controlled experimental conditions, constitutes the very large area of electroanalytical chemistry. Much recent effort has been devoted to the development of electrochemical sensors. These are systems which miniaturize and confine complex solution chemistry and electrochemistry to small probes whose sensitive and reversible responses to specific solution components allows their concentration to be determined. A particularly attractive type of sensor is that based on the specific reactions of enzymes.
Implicit in the demonstration and implementation of Faraday’s laws are the concepts of atomicity and the nature of ions and electrons. Evidently charges transferred through solutions and across electrode/solution interfaces are ‘atomic’ in nature.
The two laws are tersely expressed as follows:
In electrolytic processes the amount of chemical decomposition is proportional to the quantity of electricity passed.
The masses of different species dissolved from or deposited at electrodes by the same quantity of electricity are in direct proportion to MR/z for each species. MR is the relative molar mass, z the change in charge number which occurs in the electrode reaction which may be represented in general terms by
Thus, when a given current is caused to pass for a given time through a series of electrolyte solutions, the extent of decomposition is always the same in terms of 1 /z moles. In this statement lies the definition of the Faraday constant as the amount of electricity required to deposit a mole of any species from solution. It has the value 96 487 C mol−1 and its units emphasize the fact that 96 487 coulombs is the amount of electricity associated with T mole of unit charges, or electrons’. For practical purposes throughout the following chapters the value of the constant is rounded to 96 500 C mol−1.
Faraday’s laws, while allowing a measure of how much electrochemical change occurs, can say nothing about how (or why) such changes take place. Further, the electron exchange reactions involve species which originate in the solution and which must travel to the electrode to become involved in the process there. In the bulk of the solution electroactive solutes will behave in a way dependent on their structure, their interaction with the solvent and the prevailing conditions. Such factors will influence the transfer of an ion through the solution to the edge of the interfacial region and its subsequent negotiation of that region. Each of these circumstances constitutes an area (or areas) of study in their own right. Their influence is augmented in the overall electrochemical processes quantitatively expressed in terms of Faraday’s laws. In short, the behaviour of ions may be considered in the bulk of a solution, at interfaces and at electrodes—three domains of electrochemistry. In Table 1.1 important features of the subject, upon which subsequent chapters focus, are listed within these domains.
The sequence of Chapters 2, 3, 4, 5, 6 and 7, concerned with principles, reflects directly the journey of an ion in solution to an electrode and its reaction there. Chapters 8, 9, 10 and 11 are concerned with the exploitation of those principles in a range of applications.
Table 1.1 Features of ele...