
This is a test
- 296 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Neuropsychology of Eye Movement
Book details
Book preview
Table of contents
Citations
About This Book
First Published in 1988. The idea for this book arose from a desire to bring together relevant information from the fields of vision research, neuropsychology, neurology, and psychiatry. The selection of topics covered by N europsychology of Eye Movements conforms to the primary areas of inquiry that currently exist. Unlike the majority of other books on eye movements, which represent proceedings of meetings, this volume is com prised of a number of critical review s of the research literature.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Neuropsychology of Eye Movement by Cris W. Johnston,Francis J. Pirozzolo in PDF and/or ePUB format, as well as other popular books in Psicología & Historia y teoría en psicología. We have over one million books available in our catalogue for you to explore.
Information
| 1 | Neurophysiology and Central Pathways in Oculomotor Control: Physiology and Anatomy of Saccadic and Pursuit Eye Movements |
INTRODUCTION
This chapter summarizes the neurophysiologic and anatomic systems that contribute to the behavioral control of ocular movements. The general trend in neuroanatomy has been to adopt a reductionist viewpoint so that most investigations have been directed toward identification of the parts of the oculomotor system with the assumption that a knowledge of the whole will be obtained from the study of the parts. While this chapter must necessarily review the components and substrates, it does so with the expectation that other chapters in this volume will rebuild the pieces in a way that makes the whole more than the sum of the parts. Leigh and Zee (1983) have written the best general review of the neurology of eye movements. This chapter abstracts and amplifies on their work. The structure of the entire visual cortex has recently been reviewed in detail (Peters & Jones, 1985). Some of the newer techniques of anatomic investigation also point toward a broader analysis. For example, assuming that regional neuronal activity is mirrored by regional cerebral blood flow, position emission tomography (PET) scans have been done following intravenous bolus injection of H2150. The results of these studies are summarized subsequently in this chapter.
OCULOMOTOR SYSTEMS1
One principal factor motivating eye movements is the need to maintain the intended image fixated on the fovea. Images moving even very slowly, at a few degrees per second, can impair vision if they are not maintained on the fovea. The image movement can result from movement of the object or from movement of the head. These movements are counteracted by the optokinetic (OKN) and vestibulo-ocular reflexes (VOR) which principally function to stabilize images on our foveas. It follows that foveating reflexes are not necessary in afoveate animals.
Other ocular reflexes change the line of sight, conjugately to move the eyes independently of the head and disconjugately to adjust binocular alignment for targets at various distances (Robinson, 1978; Walls, 1969). The disconjugate movements are convergence and divergence.
From a functional standpoint, eye movements can be classified into six broad categories. The first of these classes of eye movements are the vestibular or VOR systems. The primary function of VOR is to compensate for head rotations with an equal and opposite slow phase ocular movement. These movements are generated from the stimulated semicircular canals which produce a signal proportion to head velocity rather than to head acceleration. The velocity signal must be integrated to produce an ocular command signal proportional to acceleration and this is done at hypothetical VOR integrators. In the past 10 years evidence has accumulated that the horizontal VOR integrator and the horizontal command integrator are located in the region of the rostral portion of the nucleus prepositus hypoglossi, at least in the cat (Cheron, Godaux, Laune, & VanderKelen, 1986). Although this is an effective mechanism in short-term head movements, in sustained head rotation the effectiveness of the VOR system tends to wane. Later as the vestibular apparatus begins to fade, the visually driven OKN system begins to function in response to the apparent rotation. The output of the OKN system has been shown to sum with the labyrinthine signals. This occurs in the vestibular nuclei (Waespe & Henn, 1977). The drive imparted by OKN is stored in the vestibular system and continues even after the lights are turned out. This is called optokinetic afternystagmus (OKAN). OKAN is of particular interest in that it allows study of OKN mechanisms uncontaminated by pursuit of the target which occurs in a lighted environment.
The third major class of eye movements are known as smooth pursuits. The principal purpose of smooth pursuits are to maintain foveal tracking. Ideally the movement of the eyes would be in a 1:1 ratio with the movement of the target. In humans the peak velocity of smooth pursuit movements is about 30° per sec. Abnormal smooth pursuits occur in various disorders including drug toxicity whereas normal smooth pursuit may help overcome normal and abnormal vestibular function that produces nystagmus. This is called the visual cancellation of the VOR.
The next major class of eye movements are the saccadic movements. Saccades are very quick phasic movements that make it possible to search fixed or oncoming visual scenes according to visual spatial coordinates. But the system must also be able to respond to the spatial coordinates of the other afferent stimuli such as tactile and auditory inputs. Saccades permit changes in the line of sight without head movement. The quick phases of nystagmus are an example of those quick phasic movements. These rapid eye movements may reach a maximal velocity of 500° per sec.
The last two classes of the basic eye movement systems are the fusional vergence and the accommodative vergence movements. These occur with a latency of 160–200 msec. Changing target distance will create a disparity between the locations of images with regard to the fovea of each eye. This disparity is corrected by a fusional vergence movement. Conversely, accommodative vergence results from the need to focus the image on the retina of either eye. Accommodative vergence, accommodation of the lens, and pupillary constriction are collectively described as “the near triad.”
Normally, the disjunctive eye movements may take up to 1000 msec and as a result they have generally been compared with the conjugate smooth-pursuit movements with which they are additive. Thus, a target that changes both distance and direction could be considered as analyzed in the brain by two fully independent systems for disjunctive and conjunctive movements. This assumption has been challenged by experiments in which an abrupt change in target distance and direction was followed by short-latency saccades that differed markedly and appropriately in the excursions of the two eyes so that the subjects made 41 to 70% of the required version change during the initial saccade. Enright (1986) has shown that vergence is facilitated by and during saccades and that about 25% of the vergence change achieved during binocular viewing could be accounted for by the accommodative (near vs. far misfocus) stimulus to one eye and that the adjustment was made prior to the eye movement.
The oculomotor system must be able to accurately adapt to long-term and short-term changes. These changes can be as external as a change in an eyeglass prescription and prism or as internal as a disease involving the brainstem. The cerebellum is probably the most important structure for recalibrating the oculomotor reflexes on the basis of the visual input. Moreover, there are direct and indirect pathways from the cerebral cortex (frontal eye field and visual cortex) and the superior colliculi to the immediate premotor regions which in turn control the ocular motor nuclei. Minor but definite disfunctions follow lesions of the frontal eye fields or superior colliculi because the inputs from these two regions are only partially mutually redundant. In monkeys saccades have longer latency after collicular ablation (Wurtz, Goldberg, & Robinson, 1980). Marked oculomotor disfunction results from combined cortical and collicular destruction (Schiller, True, & Conway, 1979; Wurtz & Goldberg, 1972).
SACCADIC EYE MOVEMENTS
Saccades include both voluntary and involuntary changes of fixation and are the fastest of all eye movements. The term saccade means “to pull,” and describes the rapid eye movements that occur during reading for voluntary changes of gaze. Saccades may be of several types. These include the quick phases of vestibular nystagmus, saccades coupled to head movements, and saccades on command. All three types of saccadic movements can be elicited in the standard neuroophthalmic examination.
The Attention Hypothesis
Two hypotheses have been advanced to explain the anatomy and physiology of saccades, the attention hypothesis, and the foveation hypothesis. The attention hypothesis was proposed by Wurtz et al. (1980) and suggests that the superior colliculus functions to shift attention to a specific spatial location and facilitates the movement of gaze toward that location and that this is done without specifying the exact location of the visual target nor the exact metrics of the saccadic eye movements required to reach the target. The foveation hypothesis implies that the superior colliculus precisely controls saccades to reach specific locations in retinotopic coordinates. The intrinsic organization of the superior colliculus does not suggest the kind of direct coupling of sensory and motor elements that would be needed to support the foveation hypothesis (Sparks, 1986). The function of saccades is directly linked to the existence of foveal vision because images that can be maintained on the fovea are seen most clearly.
Previous models of saccades hypothesized that the target localization was retinocentric, but current thinking is that the target localization is performed with regard to the spatial coordinates relative to the head. In these spatial models at least three inputs are required to initiate a visually directed saccade: (1) The retinal error signal (the direction and distance of the target from the fovea, (2) The position of the eyes in the orbit (obtained from the proprioceptors or oculomotor efference copies or both), and (3) information about any head movement. The retinal error signal is thought to be combined with the current eye position in the orbit to determine the target position with respect to the head. The motor-error signal is produced after a delay in which the current eye position is subtracted from the target position in head coordinates (Sparks, 1986). The superior colliculus can support the retinal spatial representation in its superficial layers and the head position coordinate representation in its deeper layers. The deeper layers of the superior colliculus also map aural and tactile space. Inputs to these layers include cues obtained from interaural time and intensity difference for acoustic localization and cues obtained from somatosensory inputs for somatotopic localization.
Anatomic Correlates
For the most of this century attention focused on a minimal schematic diagram connecting the frontal lobes via the descending frontobulbar pathways to the caudal pons for controlling horizontal saccades and to the rostral mesencephalon for controlling vertical saccades (Fig. 1.1). More recently attention has focused on schematic and computer generated models for the control of saccades. These models in turn required locating the modulating functions in anatomic locations other than those stressed in the minimal schematic diagram. The anatomic locations that have had renewed interest are the parietal lobe, superior colliculus, and the pontine paramedian reticular formation (PPRF). The PPRF is located just ventral and lateral to the medial longitudinal fasciculus and extends from the abducens nucleus to just caudal to the trochlear nucleus. The PPRF is the presumed location of many premotor saccade related neurons (VanGisbergen, Robinson, & Gielen, 1981). We discuss these in greater depth later in the chapter.
The stimulation of frontal cortical area 8 in monkeys has been shown to elicit contralateral eye movements with a latency of about 25 msec. These movements are saccadic in nature. In addition the frontal eye fields project to the basal ganglia, thalamus, pretectal retions, superior colliculus, and certain portions of the mesencephalic and pontine reticular formation (Leichnetz, 1981). Stimulation of specific areas in the frontal cortex elicit saccades of different sizes and directions. Stimulation of both frontal eye fields at corresponding sites elicits a purely vertical saccade presumably because of cancellation of the corresponding horizontal components.
Using PET scans the frontal eye fields of humans were found to have increased regional blood flow when the subject was performing saccades looking between two fixed targets, looking at alternately illuminated targets, and imagining looking at memorized targets. Similar activity was detected in the anterior portion of the pre-Rolandic supplementary motor area on the medial surface of each hemisphere when the same visual tasks were performed. The supplementary motor seemed to parallel the function of the frontal eye field and may relate to the readiness to move. Activity was identified in the primary visual cortex and lateral visual association areas only with the real targets but not with the imaginary targets. This suggests that the lateral visual association areas are not required for the generation of untargeted saccades. Several regions that have been associated with voluntary eye movements lacked activation during the PET scan study described here. This may be due to lack of resolution of the scan, lack of sufficient regional change in blood flow, lack of neurons participating in a task or to lack of a target having more interest than a light-emitting diode. No change was recorded in the inferior parietal lobule which is a region of enhanced electrical activity during visually guided saccades in primates (Fox, Fox, Raichle, & Burde, 1985; Mountcastle, Andersen, & Motter, 1981). There are probably methodologic limitations as the PET scan also failed to show activation in the basal ganglia and the superior colliculi regions well established as involved in the generation of saccades.
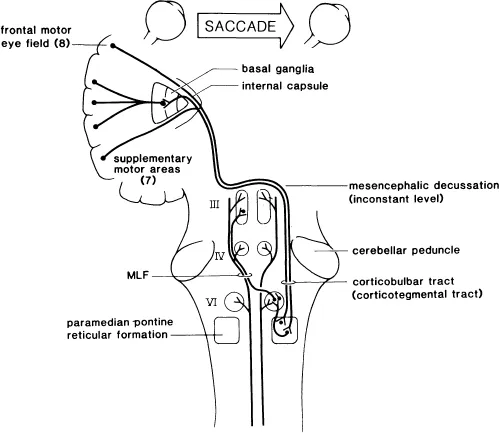
FIG. 1.1. Minimal schematic diagram for the supranuclear control of saccadic horizontal gaze movements. The target is contralateral to the frontal eye fields. For simplicity any bicortical or occipital influences on the saccadic system have been omitted. The paramedian pontine reticular formation (PPRF) extends more rostrally than is shown in the diagram. The PPRF is postulated to contain the functional “pulse generator” with “burst” neurons in its more rostral regions and the functional “neural integrator” with “burst, tonic, and pausing” neurons in its more caudal regions. The medial longitu...
Table of contents
- Front Cover
- Half Title
- NEUROPSYCHOLOGY AND NEUROLINGUISTICS
- Title Page
- Copyright
- Contents
- Preface
- Introduction
- 1. Neurophysiology and Central Pathways in Oculomotor Control: Physiology and Anatomy of Saccadic and Pursuit Eye Movements
- 2. Normal Lifespan Developmental Changes in Saccadic and Pursuit Eye Movements
- 3. Dyslexia: The Role of Eye Movements in Developmental Reading Disabilities
- 4. Effects of Psychoactive Drugs on Ocular Motor Behavior
- 5. Eye Movement Abnormalities in Schizophrenic and Affective Disorders
- 6. Eye Movements in Progressive Cerebral Neurological Disease
- 7. Oculomotor Disturbances in Hemispheric Disease
- 8. Eye Movement Behavior in Aphasia
- 9. Eye Movements in Visual Hemi-Neglect
- 10. The Last Chapter: All About What Has Gone Before
- Author Index
- Subject Index