![]()
Chapter 1
THE ECONOMICS OF METAL PRODUCTION
1.1. THE COST OF METALS
Many new materials for use in manufacture have been invented, developed and produced in the 20th century. Plastics, composites and ceramics are now well established for a wide range of applications. But metals, together with wood and concrete, still retain a dominant position in terms of the tonnages which are used annually. Although the new materials may provide a better combination of physical and mechanical properties for some applications, or simply improved aesthetic appeal, they have not made significant impact on the quantities of metal used. If wood and cement are excluded, the annual production of metals exceeds the quantity of all other construction and manufacturing materials. Steel continues to dominate the market; in tonnage terms the world production of just under 800 million tonnes of steel per annum is 93% of the total output of metals and is roughly five times the total production of composites, plastics, cermets and all the other metals. In contrast, only 11 million tonnes of plastics were made in 1989.
A number of factors account for the predominance of metals including properties (strength, toughness, corrosion resistance, electrical and thermal conductivity, etc.), formability and surface appearance, but a major factor is the cost to the customer. The metal extraction industries are well established throughout the world and have attained a high degree of efficiency, which is still improving, thus holding down production costs and maintaining or reducing the real costs when corrected for the effects of inflation. But metals will only retain their preeminent position for as long as the total cost (including maintenance and replacement cost) of artifacts produced from them is lower than that for artifacts produced from alternative materials. Over the past few decades there has been extensive replacement of metals by plastics in domestic appliances. There is increasing substitution of plastics, composites and ceramics in motorcar and aerospace vehicles. The metallurgical industries are making major efforts to retain their share of the market and their success is demonstrated by the continuing rise in the total consumption of metals.
This chapter examines the major metallurgical factors which account for the cost of the production of metals and indicates how these influence the selection of the extraction and refining routes. The major manufacturing routes for the principal engineering metals are briefly described. This provides a background for exploration in subsequent chapters of the scientific principles and constraints inherent in the various processes, consideration of which can lead to further improvements in efficiency of manufacture.
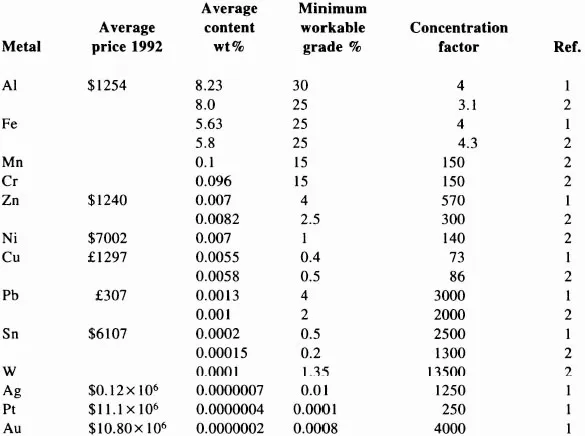
TABLE 1.1
The Price of High Grade Metals and their Average Concentrations in the Earth’s Crust
The selling price of metals (i.e., cost of production plus profit) varies over a very wide range from gold and the precious metals at one extreme to iron at the other. The price quoted for any one metal can also vary over a wide range, depending on the purity or the value of specific properties (e.g., the electrical conductivity of copper) and the cast or wrought form in which it is marketed. Many metals are traded through Metal Exchanges, where the prices are fixed daily in response to supply and demand. The average selling prices throughout 1992 for some high-grade metals are listed in Table 1.1. The prices given for the base metals are the average settlement prices on the London Metal Exchange where, by tradition, the metals are quoted in dollars (U.S.) per tonne except for Cu and Pb which are always quoted in pounds (U.K.) per tonne. The equivalent annual price in dollars is about $2270 for Cu and $537 for Pb. Iron is not normally traded through an Exchange and is not traded in bulk as a high purity material equivalent to the high-grade nonferrous metals. The nearest equivalent is very low carbon, unalloyed steel containing about 99.98% iron, which is valued at about $350 per tonne in ingot form.
Inevitably, the price of a metal is high when it first becomes available and falls as the scale of production is increased. For example, in antiquity when silver was first available in usable quantities, it was valued almost as highly as gold. Iron held a similar position with the Pharaoh’s of ancient Egypt. In more recent times, aluminum was valued at about £ 130/kg when it was exhibited at the Paris Exhibition in 1855. The real cost of metals, after correction for inflation, has fallen steadily during the 20th century as scientific and technological knowledge has contributed to closer control of the processes and as the scale of production has increased. But the price of one metal relative to another has fluctuated widely during that time. Thus, in 1951, high-grade tin sold for over five times the price of copper. By 1990 the price differential had decreased to a factor of a little over 2 but had increased again one year later to about 2.5 and to 2.7 in 1992. So, a scale of relative prices is appropriate only to the moment in time to which it refers. It is a useful concept, however, against which the factors affecting cost can be considered.
1.2. MINERAL DEPOSITS
1.2.1. Average Concentrations of Metals
The earth is the source of almost all the metals used by mankind. Large quantities of some metals have, over time, dissolved to form aqueous solutions in surface waters and are now distributed throughout the seas but, with the exception of magnesium, metals are not normally produced from seawater. The major source is the thin layer of continental crust of the earth. The average amounts of some common metals within this surface layer are listed in Table 1.1 in order of decreasing concentration. The reference numbers identify the source of the data listed in the last three columns of the table.
There is poor correlation between price and the average concentration of the metals in the earth. From its position in the table one would expect, for example, that Pb would be more expensive than Cu, Ni or Zn, but it is the lowest cost metal in this group. In fact, metals would be too expensive for general use if they had to be produced from earth containing only the average amounts of the elements. With the exception of Al and Fe, most would have production costs similar to, or even higher than, the present price of gold. Fortunately for mankind, natural phenomena have produced local areas of enrichment of metallic minerals in the form of ore bodies and these are the source of the metals. The enrichments have been produced by the selective transport of metals from the core of the earth by igneous intrusions and by the weathering of the surface rocks to produce selective accumulation of metals in sedimentary deposits.
1.2.2. Igneous Intrusions
The starting point in the formation of an ore body is an intrusion of molten magma from the core of the earth into the surface mantle of solid rock. If the intrusion reaches the outer surface as a volcanic eruption, the volatile species escape into the atmosphere and there is little time for the remaining minerals to segregate before the lava solidifies. When the intrusion does not reach the surface, however, the volatiles cannot escape and fractional solidification occurs from both the gas and the liquids as the temperature falls. Some metals solidify from the magma as oxides combined with other inorganic compounds (e.g., as silicates) or as sulfides that are immiscible with the oxides. Differences in density may cause preferential settling and concentration of the heavier minerals such as chromite (FeO·Cr2O3), ilmenite (FeO·TiO2) and magnetite (Fe3O4) as in the formation of the Swedish magnetite deposits. Metals that form oxides, sulfides, etc. with lower melting points remain in the melt at this stage.
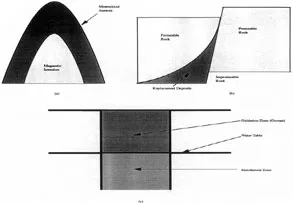
The solubility of a gas in a liquid decreases as the temperature falls. Consequently, the volume of gas associated with the intrusion increases as the magma cools. The heat released from the magma is transferred to the surrounding bedrock and volatile species are also released from the rock as it reacts with the hydrothermal gases. The sum effect is an increase in the pressure and in the fluidity of the liquids, forcing the remaining liquid magma and the associated gases away from the intrusion into the cracks and pores of the bedrock. The minerals that crystallize from the magma and the hydrothermal solutions at this stage appear as seams, lodes or veins infilling the cracks and as fine disseminations infilling the original pores in the rock (Figure 1.1a). In general, Au, Sn and W tend to occur in the aureole nearest the intrusion, with Cu in the next zone and Ag, Hg, Pb and Zn in the outer zone. Frequently, however, an intimate mixture or a solid solution of minerals is found in the same zone. The sulfides of Cu, Pb and Zn often occur together and, similarly, two or more of Fe, Co, Cu and Ni sulfides may be found in an intimate mixture. The latter type is found in the Ni deposits at Sudbury, Ontario.
Magmatic intrusions are formed only rarely today. The vast majority of igneous mineral deposits were formed hundreds of millions of years ago when the crust of the earth was less stable. Many deposits were formed when molten rock was formed by the collision of tectonic plates, between 200 and 400 million years ago, to form the present distribution of the continents.
1.2.3. Sedimentary Deposits
Ore bodies are also formed by weathering of bedrock. The action of frost, rain, wind and, particularly, the movement of glaciers during the ice ages resulted in the erosion of the surface rocks. Fragments of rock released by this action were ground down to finer sizes by fast-flowing rivers and by tidal action. The particles suspended in the water were deposited when the velocity of the transporting water decreased, as when a river emerged from hills onto a plain, or discharged into a lake or the sea. The zone of deposition varied with the size and density of the particles. For particles of similar size, those of highest density were deposited nearer to the source while the lighter particles were carried farther away. This process is still continuing and can often be observed in river beds. Placer deposits are formed in this way. The miners, panning for gold in the days of the “gold rush,” were exploiting this method of concentration and panning is still practiced today in some parts of South America. It was the original source of Cornish tin, which was first recovered from river beds some 2000 years ago and about two thirds of the tin extracted around the world is recovered from placer deposits.
FIGURE 1.1. Formation of ore deposits. (a) Molten intrusion; (b) replacement or sedimentary deposit; (c) chemical weathering.
The concentration of minerals is further enhanced by dissolution of components of the bedrock in surface waters. Carbon dioxide dissolves in water to form a weak solution of carbonic acid which, in turn, can dissolve small amounts of some metallic minerals. The minerals are deposited from solution when the acidity of the water is decreased by further reaction. The ochre color of some rivers due to iron in suspension and in solution is a manifestation of the process. The amount of mineral transported per liter of water is minute, but the cumulative effect over hundreds of years has often resulted in massive accumulation of minerals. Subsequent burial of the deposits by silt, followed by temperature and pressure changes in the earth, has converted the deposits into a sedimentary rock.
Although this is the most common way in which sedimentary deposits are formed, other mechanisms can also produce mineral enrichments. For example, surface water may percolate down through the rock strata and carbonic acid contained in the water can dissolve the bedrock, forming soluble inorganic salts. Residual deposits are formed when the impurities have been leached by acidified water to leave a massive residue of an insoluble mineral. Bauxite deposits are formed in this way, iron oxides and silica being leached from the rock to leave a deposit rich in alumina which is mined for the production of aluminum. Or the dissolution of the rock may be accompanied by the deposition of metallic minerals to form replacement deposits. Thus, water penetrating down faults in beds of limestone in Cumberland, Great Britain, dissolved caverns where the limestone rested on a bed of impervio...