
- 388 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
A core task of engineers is to analyse energy related problems. The analytical treatment is usually based on principles of thermodynamics, fluid mechanics and heat transfer, but is increasingly being handled computationally.
This unique resource presents a practical textbook, written for both undergraduates and professionals, with a series of over 60 computer workbooks on accompanying downloadable resources.
The book emphasizes how complex problems can be deconstructed into a series of simple steps. All thermophysical property computations are illustrated using diagrams within text and on the downloadable resources.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 Introduction to heat transfer
Heat transfer is a naturally occurring phenomenon. Its occurrence takes place by the virtue of temperature difference. The applicability of laws that govern heat transfer are universal and, as shall be demonstrated later in this chapter, even casual observation will show that the heat exchanges between heavenly as well as terrestrial bodies are incessant. The application of the principles of heat transfer is truly universal.
Heat transfer is a highly developed discipline and its processes are based on sound physical laws. There is a great deal of empiricism within the field of study of convection (heat transfer by fluid motion), but, even so, the basic analysis is still derived from well-established laws. The second law of thermodynamics states that ‘it is impossible to transfer heat from a body at a lower temperature to a body at higher temperature without the use of an external agency’. Furthermore, all three modes of heat transfer – conduction, convection, and radiation are governed by the respective laws due to Fourier, Newton, and Stefan–Boltzmann.
From a very early age we experience the everyday occurrence of heat transfer. The phrase ‘getting one's fingers burnt’ has indeed become synonymous with bad experience. We learn very early on that woollen clothing provides better insulation than cotton fabric, soaking in the sun provides heat or blowing over our hands while rubbing them on a cold winter morning keeps them warm. Even subconsciously we develop the ‘knack’ of shivering of limbs to provide warmth to our bodies.
In the following sections, more elaborate examples are provided to demonstrate the universality of heat transfer processes and the applicability of its laws. For obvious reasons, also included herein, are examples taken from engineering practise.
1.1 The cooling down of the universe and galaxies
Barrow and Tipler (1986) have proposed an interesting ‘Anthropological cosmological principle’ for the Universe. Their postulation is based on the premise that ‘observers will reside only in places where conditions are conducive to their evolution and existence’. In their above referred book Barrow and Tipler argue that in order to create and build the life-forming elements – carbon, nitrogen, and oxygen – the simpler elements, hydrogen, and helium were thermally treated in the interior of stars following the Big Bang event. The authors argue that the requirement that enough time pass for cosmic expansion to cool off via virtue of radiant heat transfer for the above processes to take place is 10 billion years. Thus, it has been shown that the rate at which heat transfer has taken place during the cooling of Universe has a direct bearing on the time of evolution of carbon-based life.
1.2 Radiating black holes
The laws of Newtonian mechanics state that, provided the mass and its distribution remains unchanged, the gravity at the surface of any astronomical body is inversely proportional to the square of its radius. The escape velocity from any given astronomical body increases with its gravity and consequently, inversely with its radius. Black holes are created when the size of a fuel-exhausted star begins to shrink. The gravity on its surface becomes stronger with the reduction in its size and hence the escape velocity increases. This process continues until a stage is reached where the escape velocity at its surface equals the speed of light. Any light entering the black hole will never escape. As a matter of fact light from other distant stars passing within the vicinity of a black hole may curl around a black hole several times before escaping or falling in.
The conventional wisdom was that a black hole never gets smaller and that nothing can come out of a black hole. However, in 1974 the physicist Hawking presented his theory of Hawking Radiation (Hawking, 1977; Ferguson, 1991). In what was at that time a controversial idea, he suggested that black holes had temperature and emitted radiation.
Based on the principles of quantum mechanics Hawking argued that pairs of particles – pairs of photons and gravitons – continually appear at the event horizon of a black hole. For the purpose of this text, and without indulging into the details of astronomical science, we may loosely consider the event horizon to be the radius at which the escape velocity is the speed of light. Two particles in a pair that start out together may then move apart. After an interval of time they come together and annihilate one another. Some of the pairs will be pairs of matter particles, one of the pair being an antiparticle. Hawking argued that particle pairs appear at the event horizon. However, before the pair meet again and annihilate each other, the one with the negative energy crosses the event horizon into the black hole. The particle with positive energy, now freed of its partnership, may now escape. To an observer at a distance it appears to come out of the black hole and this is known as Hawking radiation.
The particle with negative energy that falls in the black hole robs it of its positive energy, thus reducing its mass and size. Hawking has thus argued that radiating black holes might continually get smaller and eventually evaporate.
Hawking's theory has been used to ascertain the entropy, temperature, and radiation emission levels of black holes. He has shown that the larger the mass of a black hole, the larger its event horizon and entropy. The greater the entropy, the lower the surface temperature and the rate of emission. He has also proposed the existence of tiny black holes that are the size of the nucleus of an atom. Such tiny black holes he argues would crackle with radiation, indeed it would be more appropriate to call them white-hot!
1.3 The Sun and its radiation
1.3.1 Solar radiation
The Sun is a medium-sized star. It emits electromagnetic energy, including X-rays, ultraviolet, and infrared radiation as well as visible light at a rate of 3.8×1023 kW. However, its attendant planets and their satellites receive only about 1 part in 120,000,000. The small part of this energy that is intercepted by the Earth, on average 1.367 kW/m2, is of enormous importance to life and to the maintenance of natural processes on the Earth's surface. The energy output of the Sun has its peak at a wavelength of 0.47 µm. Solar radiation emerges at the photosphere. Detailed spectral studies reveal that the composition of this region is about 90% hydrogen, 9.9% helium, and a small admixture of heavy elements (Encyclopaedia Britannica, 1997).
1.3.2 Sun's corona
The temperature at the Sun's surface, that is, the photosphere, is around 6000°C. However, moving away from the photosphere does not lower the temperature. In the 3000-km zone between the photosphere and the corona, the temperature jumps from 6000°C to over a million celsius. In apparent defiance of common sense, the further away you go from the Sun's surface into the solar atmosphere, the warmer the gas becomes! There have been many theories postulated that try to explain the extraordinary warmth of the Sun's corona. The more popular of these fall into three categories, namely miniature solar flares, atmospheric waves, and electrical dissipation. A detailed discussion of these theories is beyond the scope of the present text and the reader is therefore directed to the NASA (1999) website.
1.4 The Earth's thermal environment
1.4.1 Terrestrial greenhouse effect
Energy from the Sun drives the Earth's weather and climate, and in turn heats the Earth's surface. The Earth radiates energy back into space. Atmospheric greenhouse gases (water vapour, carbon dioxide, and other gases) trap some of the outgoing energy, retaining heat somewhat like the glass panels of a greenhouse, as shown in Fig. 1.4.1.
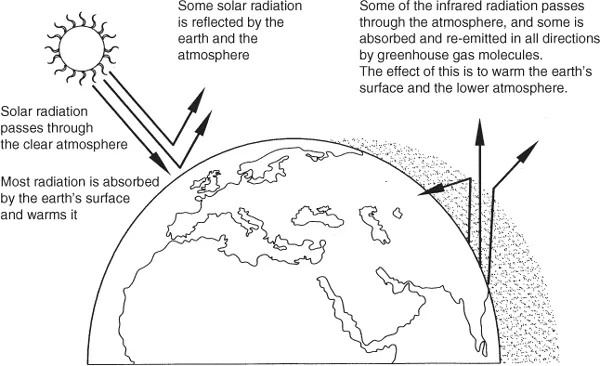
Figure 1.4.1 The terrestrial greenhouse effect.
Without this natural ‘greenhouse effect’, temperatures would be much lower than they are now, and life as known today would not be possible. However, problems may arise when the atmospheric concentration of greenhouse gases increases. Since the beginning of the industrial revolution, atmospheric concentrations of carbon dioxide have increased by nearly 30%, methane concentrations have more than doubled, and nitrous oxide concentrations have risen by about 15%. These increases have enhanced the heat-trapping capability of the Earth's atmosphere. Sulphate aerosols, a common air pollutant, cool the atmosphere by reflecting light back into space. However, sulphates are short-lived in the atmosphere and vary regionally.
As a result of the increase in greenhouse gas concentrations, global mean surface temperatures have an increasing trend. The twentieth century's 10 warmest years all occurred in the last 15 years of the century. Of these, 1998 was the warmest year on record. The snow cover in the Northern Hemisphere and the area of floating ice in the Arctic Ocean have both decreased. Increasing concentrations of greenhouse gases are likely to accelerate the rate of climate change. Scientists expect that the average global surface temperature could rise by 0.6–2.5°C in the next 50 years, and 1.4–5.8°C in the next century.
Most greenhouse gases occur naturally in the atmosphere, while others are produced as a resul...
Table of contents
- Front Cover
- Half Title
- Title Page
- Copyright
- Dedication
- Contents
- Highlights of the book
- Preface
- Acknowledgements
- Disclaimer and copying policy
- Contents of companion CD-ROM
- Workbooks available from website
- Notation
- 1 Introduction to heat transfer
- 2 Numerical and statistical analysis using Microsoft Excel
- 3 One-dimensional, steady-state conduction
- 4 Multi-dimensional, steady-state conduction
- 5 Transient conduction
- 6 Introduction to convection
- 7 Forced convection
- 8 Natural convection
- 9 Thermal radiation
- 10 Multi-mode heat transfer
- Appendices
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Heat Transfer by Kubie Jorge,Tariq Muneer,Grassie Thomas in PDF and/or ePUB format, as well as other popular books in Business & Decision Making. We have over one million books available in our catalogue for you to explore.