![]()
Section III
Applications
![]()
13 | Integrated Multi-Organoid Dynamics Aleksander Skardal, Mahesh Devarasetty, Sean Murphy and Anthony Atala |
CONTENTS
13.1 Introduction | |
13.2 Single versus Multiple Organoid Function | |
13.2.1 Cancer | |
13.2.2 Drug Testing/Toxicology | |
13.2.3 Disease Modeling | |
13.3 Biofabricating a Highly Physiologically Accurate Multi-Organoid Platform | |
13.3.1 Overview of ECHO Platform Organoids | |
13.3.2 Microfluidic Hardware Integration | |
13.3.3 Common Media Development | |
13.3.4 Miniaturization and High-Throughput | |
13.4 Current and Future Applications | |
13.4.1 Drug Testing | |
13.4.2 Precision Medicine | |
13.4.3 Multi-Organoid Cancer Systems | |
References | |
13.1 INTRODUCTION
There is a critical need for improved systems to model the effects of chemical and biological agents on the body.1,2 Currently, animal models serve as gold standards for testing, but the drawbacks associated with such models are high costs and uncertainties in interpretation of the results. Interspecies differences and variability means that animal models are often poor predictors of human efficacy and toxicology. In vitro systems that use human tissues would be preferable; however, for these systems to serve as tools that reflect human biology, key physiological features and toxicology endpoints need to be included in their design for informative and reliable efficacy, pharmocokinetic, and toxicity testing. Traditional in vitro 2D cultures, currently the norm for early drug compound screening, fail to recapitulate the 3D micro-environment of in vivo tissues.3,4 Tissue culture dishes have three major differences from native tissue microenvironments: surface topography, surface stiffness, and most importantly, a 2D rather than 3D architecture. As a consequence, 2D culture places a selective pressure on cells, substantially altering their original phenotypic properties. Drug diffusion kinetics are not accurately modeled in 2D tissue culture, drug doses effective in 2D are often ineffective when scaled to patients, and the lack of cell-cell/cell-matrix interactions in 2D often lead to loss of cell function.3,5,6 Instead, “organ-on-a-chip” devices that can simulate 3D tissue architectures and physiological fluid flow conditions are better options. Such devices are capable of producing rapid, reliable predictions of biological processes, disease states, and drug and toxicology responses. Additionally, these engineering platforms benefit by having reproducible hardware, a simple scale up transition, high throughput assay capability, automation potential, and control over physical environmental conditions such as fluid flow and shear stress. These microengineering and microfluidics technologies have resulted in advanced studies in creating and culturing 3D human tissue on a chip.7 Currently, many organ-on-chip systems exist,8,9 as well as several on-chip disease models.8 By biofabricating the respective cell type or combining several cell types into 3D tissue constructs, these model organs can be viable for longer periods of time and are cultured to develop functional properties similar to the native tissues. The next challenge is to combine several organs on the same microfluidic device to model a simple organism-on-a-chip for drug and therapeutic studies. Ultimately, in the human body, tissues and organs are interconnected and interdependent on one another.
13.2 SINGLE VERSUS MULTIPLE ORGANOID FUNCTION
In vitro models that accurately recapitulate human tissues and model disease are limited, and fewer still exist in which multiple tissues are represented in an integrated fashion. This is an important technological limitation as tissue and organ development and function within the body does not occur in isolation, and it is essential that organs receive vascular, neural, metabolic, and hormonal signals and support for normal tissue function. In drug screening, for instance, toxic effects in secondary tissues can be as important as effects at the target site. If undetected, these effects can lead to an unnecessarily high rate of failure or withdrawal due to side effects. Likewise, in cancer metastasis, in which malignant tumor cells migrate from one location to another, multiple tissue or organ sites and a circulatory system (vascular or lymphatic) are involved. As such, while useful for many applications, single organoid models have limited efficacy for recapitulating the complex physiological interactions between multiple tissues that occur often in the human body. Instead multi-organoid platforms are necessary. Here, we describe several examples of multi-organ interactions that demonstrate the importance of considering deployment of multi-organoid platforms over single organoid systems.
13.2.1 CANCER
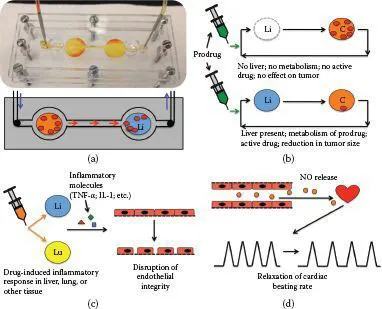
As described earlier, one important phenomenon that requires a multi-organoid approach for recapitulating it in vitro is modeling of cancer metastasis. In metastasis, cells in a tumor typically under go epithelial-to-mesenchymal (EMT) transition, proliferate rapidly in the primary tumor site, intravasate through endothelial cells into the blood stream or lymphatic system, after which they extravasate and colonize a downstream tissue. Currently, few in vitro systems exist that employ a multi-organoid approach. However, they are in development, and our laboratory has demonstrated that it is possible to recapitulate metastasis in vitro, albeit in a reductionist manner. Our metastasis-on-a-chip platform was devised to allow tracking of tumor cell metastasis from a colon organoid to a liver organoid within a simple microfluidic device (Figure 13.1a). We demonstrated that metastatic HCT116 cells were able to migrate and disseminate out of the colon organoid into the circulating media system and engraft in the downstream liver organoid. Conversely, nonmetastatic cell type SW480 proliferated at the primary site but never migrated to the liver. Using this platform we performed drug screens with common chemotherapy agents and also showed that we could manipulate the physical tumor microenvironment through hydrogel chemistry, altering environmental elastic modulus, which could stunt or accelerate tumor cell migration from tumor foci into the surrounding organoid space. We are currently advancing this system in several ways. We have recently integrated endothelial barriers in order to support intravasation and extravasation. We have also begun adding additional downstream organoids, with a goal being to explore what is more important in metastasis—proximity and location of the downstream site, or the cellular and extracellular matrix composition of the downstream site.
FIGURE 13.1 Multi-organ interactions. (a) Metastasis of tumor cells from one organ/organoid site to another, demonstrated in vitro in a metastasis-on-a-chip device in which colorectal carcinoma metastasizes from the colon to the liver. (b) Reliance of a prodrug (e.g., an anticancer 5-fluorouracil prodrug) on liver metabolism to activate the drug to generate a positive effect. (c) Inflammatory molecules secreted from organs such as the liver and lung can affect downstream integrity of vascular endothelium by disrupting the barrier function of endothelial cells. (d) Nitric oxide release from the vasculature in high levels can result in relaxation of cardiac beating rates.
13.2.2 DRUG TESTING/TOXICOLOGY
A broad area of importance is how multiple organs and tissues respond to administration of particular drugs. A variety of examples exist that demonstrate this concept. For example, 5-fluorouracil (5-FU) is one of the many common chemotherapy agents employed in treating colorectal cancer. Unfortunately, 5-FU can induce a variety of detrimental side effects in patients. In an attempt to reduce toxicity, several 5-FU prodrugs have been developed. These prodrugs are inactive in the native form, only becoming active after metabolism, generally in the liver. Consequently, without including a metabolically active liver organoid in in vitro 5-FU prodrug drug studies, results would be essentially meaningless. Building a platform with a liver and a tumor would allow metabolism of the prodrug followed by assessment of the activated drug on the tumor (Figure 13.1b).
13.2.3 DISEASE MODELING
An additional example incorporates a variety of organs and the vasculature. There are many drugs that are known to cause inflammatory responses in different tissues. For example, large doses of the analgesic acetaminophen (i.e., Panadol, Tylenol) cause inflammation and toxicity in the liver. Similarly, chemotherapeutics such as bleomycin cause inflammation, toxicity, and irreversible fibrosis in the lungs. In both cases, toxicity and cell death results in release of inflammatory molecules such as TNF-α and interleukin-1 into the circulation—both of which, in turn, can cause disruption and loss of integrity in the vascular endothelium (Figure 13.1c), similar to a response to histamine. Likewise, insult to the vasculature can lead to release of nitric oxide, which at high enough levels can change the beating profile of the heart (Figure 13.1d). These are just a few examples of significant downstream impacts of upstream drug treatments. Integrated multi-organoid model systems are required to detect these complex and multi-organ drug effects in a predictive and physiologically relevant manner.
13.3 BIOFABRICATING A HIGHLY PHYSIOLOGICALLY ACCURATE MULTI-ORGANOID PLATFORM
Our team’s advancement of biofabrication and tissue engineering techniques has enabled formation of artificial constructs that model the organization of diverse tissues and tumors and can be serialized and addressed with fluidic technologies. One of the main problems in the implementation of tissue-engineered 3D model systems, whether for cancer applications, drug development, or toxicology, is that they simply do not sufficiently mimic their in vivo human counterparts. To provide a model system that can be employed to predict how a drug will affect a patient, or how malignant a tumor will be, model tissue constructs necessitate a baseline level of physiological accuracy. This includes morphology and multicellular organizations, functionalities such as protein synthesis and secretion, metabolic rates, drug metabolism, response to drugs and their metabolites, and long-term stability and viability. Our team is at the forefront of in vitro model development and implementation and has developed a portfolio of 3D tissue models. Crucially, these discrete “organoids” reproduce functionality of in vivo systems; for example, they synthesize and secrete typical biomarkers, respond to toxins, and metabolize drugs. Snapshots of these advanced systems are given below.
Our team was fortunate to secure a competitive Department of Defense contract through the Defense Threat Reduction Agency in 2013 to develop an integrated body-on-a-chip system for use in bioweapon and chemical weapon assessment and countermeasure development. Our team, lead by Dr. Anthony Atala at the Wake Forest Institute for Regenerative Medicine, led a multi-institutional collaboration between investigators at Brigham & Women’s Hospital, Harvard Medical School, Johns Hopkins University, the University of Michigan, and the Edgewood Chemical and Biological Center ...