![]()
Part I
Generality on arsenic, fluoride and uranium
![]()
CHAPTER 1
Fluoride, uranium and arsenic: occurrence, mobility, chemistry, human health impacts and concerns
Alberto Figoli, Jochen Bundschuh & Jan Hoinkis
1.1 INTRODUCTION
Mainly due to global population growth the demand for potable water is continuously rising. According to the United Nations, world population is projected to reach 9.6 billion by 2050 with most growth in developing regions, especially in Africa (UN, 2013). During the same period, the population of developed regions will remain largely unchanged at around 1.3 billion people (UN, 2013). This forecast highlights well the need for safe drinking water especially in less developed regions, where the population is expected to grow significantly. About 97% of the freshwater reserve is stored in aquifers, which makes the groundwater the largest global freshwater resource. That resource caters to the need of a population of over 1.5 billion (Jacks and Battacharya, 2009). When comparing this freshwater to surface water quality many advantages turn out. Groundwater is generally free from pathogenic bacteria and viruses and has far lower concentrations of organic matter. However, a variety of organic and inorganic contaminants have been identified in ground-water that are potentially toxic to humans or animals (Hoinkis et al., 2011). The origin of these contaminants is on the one hand naturally occurring through mobilization from the rocks and minerals through physical, chemical, and microbiological processes into the groundwater while on the other hand uniquely human sources like pesticides, fertilizers or industrial and mine waste discharge are other sources.
Rapid and intensive industrialization has generated large volumes of aqueous wastes containing dangerous materials, such as heavy metals and metalloids. Water contamination by heavy metals, metalloids and other minor and trace elements such as fluoride constitute a big global health hazard (An et al., 2001; Mulligan et al., 2001) as they can be toxic and carcinogenic even at very low concentrations, and, hence, usually pose a serious threat to the environmental and public health (Liu et al., 2008; Vilar et al., 2007). During traditional wastewater treatment, most heavy metals (e.g., lead, chromium and cadmium) and metalloids (e.g., arsenic (As)) pass unhindered through the treatment process, which is mainly due to their occurrence in trace amounts. In fact, little to no attention has historically been given to metals and metalloids in wastewater treatment plants.
Natural sources (volcanic emission, weathering of rocks and microbiological activity), release of geogenic contaminants through mining and anthropogenic sources (e.g., burning of fossil fuels, use of arsenical pesticides and herbicides, etc.) are responsible for highAs concentrations in water in many parts of the world (ATSDR, 2007). Inthe affected areas, As concentrations in ground water are generally found in the range of 100–2000 μg L−1 On the other hand, the potential sources for high concentration of F− in water are dissolution of F− bearing rocks under favorable natural conditions and/or discharge of F− contaminated wastes from the industry (Mohapatraet al., 2009). Moreover, like As and F, U is also distributed in the environment due to natural (weathering of rock) and anthropogenic (mining, nuclear power production and phosphate fertilization) sources and leaves a very high impact on the environment, which is a latent risk factor for both human and animals (Langmuir, 1997; Oliver et al., 2008).
As a result of the high concentration of these chemical species in groundwater, an adequate treatment is required for removal of these contaminants before supplying it for human consumption.
Various chemical treatment technologies have been applied to remove these ions from drinking water sources, including ion exchange, metal oxide based adsorption and coagulation. However, these methods alone are insufficient to remove the contaminants below the Maximum Contaminant Limit (MCL) and therefore are better to be used as a pretreatment step (Favre-Réguillon et al., 2005; Mondal et al., 2013).
In fact, the presence of such inorganic arsenic (As(V/III)), fluoride (F−) and uranium (U(VI)) species (mostly ions) in groundwater (and to less extent in surface water) is a critical global issue, and has created severe health impacts for decades. Bioaccumulation and adverse effects on human health by intake of these ions via drinking water have been well documented (e.g., Fawell et al., 2006; Orloff et al., 2004; Smedley and Kinniburgh, 2002).
The aim of this book is to describe, analyze and bring to the attention the existence of different types of membrane processes, which could be successfully applied for the removal of toxic metals from water. In particular, the removal of As, U and F− fluoride will be taken into consideration as specific cases.
This introductory chapter for this volume provides basic information on the occurrence and chemical species of As, U and F− in freshwater resources (predominantly groundwater), their release from rocks and sediments and mobility as well as the principal health impacts, which occur due to human uptake through drinking water or through the human food chain. The chapter provides only simplified insight into these topics as far as this knowledge is needed for selecting the most appropriate technology and design for removal of these trace contaminants from drinking and irrigation water and provide the reader with knowledge of the global importance of contamination of freshwater resources with these geogenic and, of minor importance anthropogenic, trace elements and related potential health impacts, which clearly demonstrate the importance of their removal through adequate treatment.
1.2 FLUORIDE
Fluorine is the lightest, reactive and most electronegative element in the halogen group of the periodic system and has a strong tendency to acquire a negative charge. Thus, it remains as a negative ion (F−) in solution (Fawell et al., 2006) and forms negative and positive complexes (e.g., dissolved [MgF]+ complexes). Soluble fluoride complexes with Al3+, Fe3+ and Si4+ have high equilibrium constants ranging from 106 to 105, but the amount of Al3+, Fe3+ and Si4+ ions is below 1 mg L−1 in most natural waters (pH 5 to 8, Eh = −200 to +200 mV) (Baas Becking et al., 1960). Graham et al. (1975) and Roberson and Barnes (1978) state that fluoride complexes with Al3+, Fe3+ and Si4+ must therefore only be considered at rather low pH-values. In areas where high fluoride concentrations are correlated with arsenic like in Arizona (Robertson, 1984) and especially in the Argentine Pampa and Chaco plains, additionally the fluoride complexes of As must be considered (e.g., HAsO3F− +H2O = F− + H+ + , pK = −46.112 and AsO3F2- + H2O = F− + , pK = −40.245) (Bundschuh et al., 2000; 2004). However, ionized and non-ionized organic and inorganic F occur in the environment.
1.2.1 Sources, release and mobility
In many regions, fluorine is a widely distributed constituent found in sedimentary porous aquifers, in porous aquifers formed by the overburdens of hard bedrock aquifers and in hard rock aquifers in concentrations beyond the WHO guideline value of 1.5 mg L−1 (Fawell et al., 2006).
The presence of F− in the environment occurs not only naturally through its presence in the earth’s crust but also due to industrial activities, such as electroplating, semiconductor manufacturing, glass making, steel production and fertilizer industries (Sujana et al., 1998; Toyoda and Taira, 2000). The release of wastewater from these industries leads to the F− pollution of surface and groundwater. The US Environmental Protection Agency (USEPA) established the effluent discharge standard of 4 mg L−1 for F− from a wastewater treatment plant (Khatibikamala et al., 2010; Shen et al., 2003). The breakdown of rocks and soils or weathering and deposition of atmospheric volcanic particles are the biggest source of F− found in the groundwater. Because they often contain abundant F−-bearing minerals crystalline rocks, especially granites, are particularly susceptible to F− build-up. Concentrations of F− in groundwater can range from below 1 mg L−1 to more than 50mgL−1.
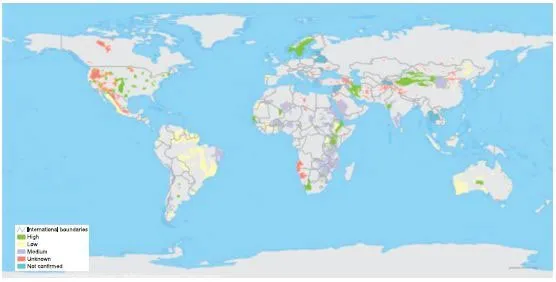
Figure 1.1. Fluoride in groundwater — Probability of occurrence (IGRAC, 2014 Environmental Data Explorer, compiled from IGRAC: Internet Site: http://geodata.grid.unep.ch/options.php? selectedID = 2241 & selectedDatasettype = 16 (accessed on 05 February 2014).
Figure 1.1 provides an overview over the probability of occurrence of the F− distribution worldwide. The known hotspots of F− with high concentrations in groundwater are found to be in Scandinavia, China, Western India, East Africa North America and South America. Despite this forecast a variety of different publications showed several other spots of high F− concentrations in groundwater. In Pakistan, Thailand, China, Sri Lanka, eastern and southern Africa high groundwater F− concentrations associated with igneous and metamorphic rocks such as gneisses and granites have been reported (Fawell et al., 2006). About half of the states and territories in India reported to have naturally high concentrations of F− in water (UNICEF, 1999). In Sri Lanka, concentrations up to 10 mg L−1 have been reported and in China fluorosis has been reported to be widely spread (Fawell et al., 2006). Since millions of people worldwide are exposed to high F− concentrations this poses a serious global health threat to the consumers.
The northern Tanzania region is known for being among the most F− affected areas worldwide. Already in the early 1980s, Nanyaro et al. (1984) reported high F− contents in some rivers, springs, alkaline ponds and lakes in northern areas of Tanzania. The F− contents found have been 12–26 mg L−1 for rivers, 15–63 mg L−1 for springs and even mg 60–690 L−1 for alkaline ponds and lakes. Also Bugaisa (1971) identified particular F− problems in groundwater of Tanzania. The concentrations found vary between 4 and 330 mg L−1. Such concentrations are extremely high compared to other F− contaminated groundwater sources. In the East-African Rift zone lavas (intrusions and ashes) and other volcanic rocks with fluorine-rich minerals are found in much higher concentrations than in similar rock types elsewhere in the world (Kilham and Hecky, 1973). Hot springs are also an important source for high F− concentrations in the groundwater. In addition, in extreme cases of evaporation of lakes coexisting with infiltration of lake water to the shallow aquifers, F− contamination of the aquifer might occur.
1.2.2 Human health effects
Fluoride toxicity can happen by a number of ways. Bhatnagar et al. (2011) noted that the impact of F− in drinking water can be beneficial or detrimental to human well-being. Small amounts in consu...