
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Biochemistry of Andrology
Book details
Book preview
Table of contents
Citations
About This Book
This textbook comprehensively covers the physiology and biochemistry of the processes in the male reproductive system. Readers will gain an understanding of a variety of general topics relevant to andrology through fourteen chapters which include information about male reproductive anatomy, sexual development, spermatogenesis, sperm-egg interactions necessary for fertilization, reproductive toxicology, congenital disorders, sexually transmitted diseases, male sterility and changes due to aging.
The book serves as a primary andrology reference for medical students studying reproductive biology.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Biochemistry of Andrology by Marco Aurélio Gouveia Alves, Pedro Fontes Oliveira in PDF and/or ePUB format, as well as other popular books in Medicine & Gynecology, Obstetrics & Midwifery. We have over one million books available in our catalogue for you to explore.
Information
Topic
MedicineBasic Aspects of Testicular Cells: Physiology and Function
João P. Monteiro*
Department of Microscopy, Laboratory of Cell Biology, Institute of Biomedical Sciences Abel Salazar and Unit for Multidisciplinary Research in Biomedicine, University of Porto, Rua de Jorge Viterbo Ferreira, 4050-313, Porto, Portugal
Abstract
Within the testes there is a considerable histological diversity, reflected in a significant variety of different circumscribed environments and cells. As is always the case regarding structures slowly forged by evolution, this translates into meaningful differences in the physiology and function for each cell type. Leydig cells are essentially known for their steroidogenic potential; Sertoli cells are known for their local support to germ cells, and peritubular myoid cells are rapidly transcending a simple structural role. They are all known to actively determine and contribute to spermatogenesis in some way. Moreover, the physiological interplay between these types of cells is known to functionally impact male fertility. However, the specific physiological mechanisms by which each cell type governs spermatozoa production are not fully accounted for, and pathways underlying the cooperative action of these cells in the process are far from being clarified. Increased knowledge regarding the function and interaction of these cells could potentially lead to important breakthroughs within the contexts of testes disease, infertility and contraception.
Keywords: Blood-testis barrier, Cell differentiation, Energetic metabolism, Hormonal regulation, Leydig cells, Paracrine regulation, Peritubular myoid cells, Sertoli cells, Spermatogenesis, Steroidogenesis.
* Corresponding author João P. Monteiro: Rua de Jorge Viterbo Ferreira, 4050-313, Porto, Portugal; Tel: +351 220 428 000; E-mail: [email protected]
INTRODUCTION
The continuous production of competent spermatozoa by sexually mature males is a complex, highly regulated multistep process implying an intricate interaction between different cooperating cell types present in the testes. Spermatogenesis is regulated by a large number of endocrine and testicular paracrine/autocrine factors. Classically, the process is perceived as being dependent on interactions between somatic and germ cells, involving the coordinated action of three main
cell lineages: supporting cells, steroidogenic cells, and germ cells. Therefore, in the testes, there is a need for a provision of differentiated cells types, and the high rate of cell turnover and differentiation displayed these organs surely is associated with that.
Sertoli cells are viewed as supporting cells in the process of spermatogenesis (they are even called “nurse cells” by some researchers). They are morphological and physiologically very distinct from all other cells present in the testes, and their importance for spermatogenesis ranges from mere physical support, to immunoprotection and nutritional support of developing germ cells. Leydig cells are mostly recognized as being steroidogenic cells. Therefore, they are known to play an important role in the hormonal regulation of the spermatogenic process and, furthermore, in ensuring an adequate definition and sustenance of male secondary sexual characteristics. Peritubular myoid cells have been relatively overlooked regarding their role in spermatogenesis. Other than just a structural role in the establishment of the basement membrane, a more active role in the process has been lately disclosed, involving relevant paracrine function. These three cell types, and their respective importance and involvement in the spermatogenic process, will be the subject of this chapter, and the relative location and organization of each cell type within the testes can be perceived in Fig. (3.1). Aspects related to their functional relationships and interactions that are meaningful for competent spermatozoa production will be discussed. Peritubular myoid cells, through the establishment of the basement membrane, preclude direct physical proximity between Leydig and Sertoli cells. However, despite actual mechanical constrictions, these cells were all shown to interact with each other locally, through paracrine mechanisms, and these interaction mechanisms have substantiated many justified studies.
LEYDIG CELLS: STRUCTURE AND FUNCTION
Leydig cells were first characterized in the 1850’s by Franz Leydig, from whom they got their name [1]. Leydig cells, located within the interstitial compartment of the testes, are arguably the most relevant cell type involved in endocrine function of the testes, being the main cells responsible for the synthesis and secretion of hormones, namely testosterone in male adults [2]. This important role of Leydig cells implies that the normal function of these cells is crucial for the reproductive activity of males. Leydig cells produce testosterone under the control of luteinizing hormone, which is produced by gonadotropic cells in the anterior pituitary gland [3] and binds to G protein-coupled receptors, activating adenylyl cyclase and therefore increasing cAMP formation [4]. Testosterone was reported to regulate a multiplicity of genes (mostly repressed) in the testes [5].
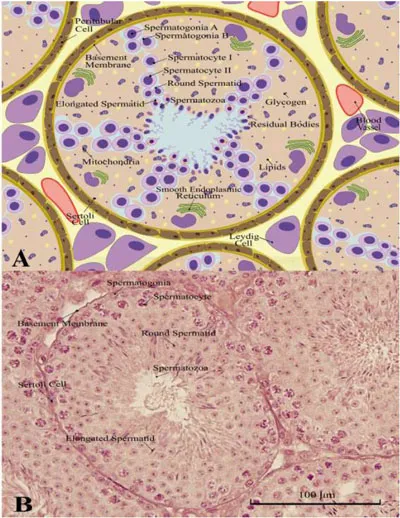
(A) Schematic representation of a transversal cut of mouse (Mus musculus) seminiferous tubules, exposing the location of Leydig cells (outside the tubules), peritubular myoid cells (disposed around the tubules, in the basement membrane), and Sertoli cells (inside the basement membrane, in close contact with germ cells, at different maturation stages). (B) Actual microscopy image of the transversal cut shown in A), where the same cells type elements are also visible.
Leydig cells have, therefore, an important role in the definition and maintenance of the secondary sexual features defining male characteristics, intermediating testes development, maintenance of spermatogenesis and general male fertility [6, 7]. Some of the processes by which testosterone impacts spermatogenesis include the sustenance of spermatogonia population, preservation of a functional blood-testis barrier (BTB), completion of meiosis by spermatocytes, establishment of adherences between elongated spermatids and Sertoli cells, release of mature spermatozoa, and the establishment of the seminiferous tubule lumen [8].
Two different populations of Leydig cells are present in mammals. Fetal Leydig cells (FLCs) and adult Leydig cells (ALCs) are present respectively in fetal and adult testes [9], but they each have particular functions and are modulated by different regulatory mechanisms [10]. FLCs and ALCs differ in terms of morphology, capability of synthesizing androgen, and response to gonadotropins and growth factors [11-13]. In morphological terms, FLCs display round to oval shapes, presenting abundant lipid droplets. They preferentially aggregate in clusters, and are encircled by a basement membrane that is rich in collagen and laminin [14]. In turn, ALCs have larger size, ...
Table of contents
- Welcome
- Table of Contents
- Title Page
- BENTHAM SCIENCE PUBLISHERS LTD.
- PREFACE
- List of Contributors
- Introduction
- Testis Physiology
- Basic Aspects of Testicular Cells: Physiology and Function
- Basic Aspects of Spermatogenesis
- Hormonal Control of Male Reproductive Function
- Male Puberty: A Triggered Biochemical Event towards Sexual Maturation
- Biochemical Events Occurring in the Epididymis
- Formation and Biochemistry of Seminal Plasma and Male Accessory Fluids
- Functional and Biochemical Aspects of Spermatozoa
- Biochemistry Behind the Journey of Spermatozoa Through the Female Reproductive Tract
- Testicular Cancer, Erectile Dysfunction and Male Reproductive Health
- Metabolic Disorders and Male Reproductive Health
- Environmental Cues and Sperm Quality
- Biochemical Changes in the Reproductive Function of the Aging Male