
- 742 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Molecules That Amaze Us
About this book
"This new book is by two knowledgeable and expert popularizers of chemistry and deals exclusively with molecules and compounds rather than with the simpler atoms and elements. It is based on the very successfulMolecule of the Month' website that was begun by Paul May fifteen years ago and to which his co-author Simon Cotton has been a frequent co
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter
1
ADENOSINE TRIPHOSPHATE
(ATP)
I DON’T GET THE JOKE?
The acronym for adenosine triphosphate is ATP, which sounds like 80p (short for 80 pence).

WHAT IS ATP?
All living things, plants and animals, require a continual supply of energy in order to function. The energy is used for all the processes which keep the organism alive. Some of these processes occur continually, such as the metabolism of foods, the synthesis of large, biologically important molecules, e.g. proteins and DNA, and the transport of molecules and ions throughout the organism. Other processes occur only at certain times, such as muscle contraction and other cellular movements. Animals obtain their energy by oxidation of the foods they’ve eaten, plants do so by trapping the sunlight using chlorophyll (see p81). However, before the energy can be used, it is first transformed into a form which the organism can handle easily. This special carrier of energy is the molecule ATP.
HOW DOES IT WORK?
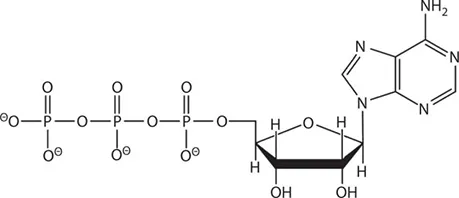
The key to how it works is in its structure. The ATP molecule is composed of three components. At the center is a sugar molecule, ribose (the same sugar that forms the basis of DNA and RNA). Attached to one side of this is a base (a group consisting of linked rings of carbon and nitrogen atoms); in this case, the base is adenine. When joined together, the sugar and base are known as adenosine. The other side of the sugar is attached to a string of three phosphate groups. These phosphates are crucial to the activity of ATP.
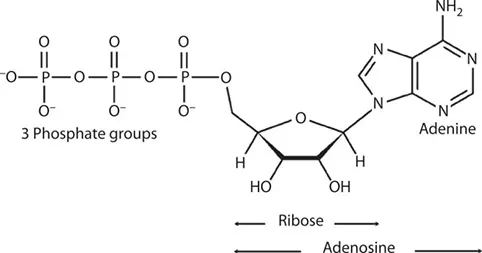
The structure of ATP showing the three components
HOW SO?
ATP works by losing the endmost phosphate group when instructed to do so by an enzyme. This reaction releases a lot of energy, which the organism can then use to build proteins, contract muscles, generate heat, etc. The reaction product has one less phosphate group and so is called adenosine diphosphate (ADP), and the liberated phosphate group either ends up in solution as orthophosphate (HPO4) or attached to another molecule such as an alcohol. Even more energy can be extracted by removing a second phosphate group to produce adenosine monophosphate (AMP).
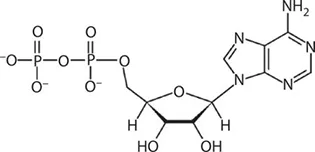
ADP
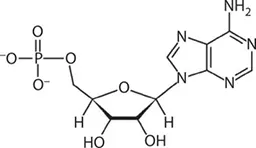
AMP
When the organism is resting and energy is not immediately needed, the reverse reaction takes place and the phosphate groups are reattached to the molecule, one at a time, using energy obtained from food or sunlight. Thus, the ATP molecule acts as a sort of rechargeable ‘chemical battery’, storing energy when it is not needed, but able to release it instantly when the organism requires it. It has been calculated that the human body contains only 250 g of ATP at any one time, which is roughly the equivalent energy of an AA battery. But it turns over more than its own weight in ATP in a day.
BUT I THOUGHT ENERGY WAS STORED AS FAT?
For long-term storage, i.e. days or years, surplus energy from food is used to synthesize long-chained fatty acids (see p27l) and stored as fat distributed around the body, or as glycogen (a form of polymerized glucose, see p193) in the liver. When energy is required by the body for a particular process, say to make a muscle contract, the stored fat or glycogen is removed from storage by enzymes and transported in the blood to the cells in question. There, it undergoes oxidation, reacting with the oxygen delivered by hemoglobin in the bloodstream (see p227), to produce the waste products water and CO2, and releasing lots of energy. The energy converts ADP and AMP back to ATP (recharging the local ‘battery’), which is then used as the power source for the cellular process. So ATP is a very temporary energy store localized within each cell.
In plants, the long-term energy store is another polymerized form of sugar called starch (see p193). In photosynthesis, the chlorophyll molecule traps energy from the sun and uses this to make ATP from ADP (see p81). The ATP is then transported to other parts of the cell which break it back down into ADP, and use the released energy to turn carbon dioxide and water into glucose, releasing oxygen. Enzymes then polymerize the glucose into starch and it is stored for later use. Hydrolysis of the stored starch using enzymes (called amylases) allows the plant to extract the glucose and use the freed energy to make ATP, which can then be used as a local energy source in cells for use in various biological processes such as growth. Amylase enzymes are also present in human saliva and allow us to digest starch. Foods that contain a lot of starch but little sugar, such as rice and potatoes, often taste slightly sweet because the amylase in saliva turns some of the starch into sugar as they are chewed.
SO WE GET ATP FROM OUR FOOD?
We get the components from food, but these are metabolized into ATP in our body. The phosphate groups are the key ingredient, and these are part of what biologists call the Phosphorus Cycle. The fact that ATP is nature’s universal energy store explains why phosphates are a vital ingredient in the diets of all living things. Modern fertilizers often contain phosphorus compounds that have been extracted from animal bones. These compounds are used by plants to make ATP. Animals then eat the plants, metabolize the phosphates, and produce their own ATP. We also eat the plants and other animals, and convert their phosphorus into our own ATP. And when we die, our phosphorus goes back into the ecosystem to begin the cycle again.
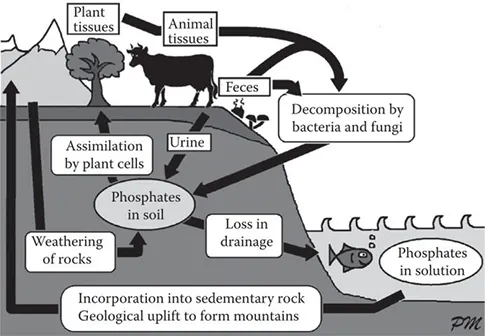
The Phosphorus Cycle in Nature
WHERE IS THE ATP MADE?
In animals, ATP is recycled in the mitochondria, which are organelles found within animals cells, and which can make up to 25% of the total volume of the cell. Mitochondria resemble smaller cells trapped within larger animal cells. They also contain their own DNA, which is different from the DNA found in the nucleus of the larger cells. This observation has led...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Foreword
- Preface
- Authors
- Chapter 1 Adenosine Triphosphate (ATP)
- Chapter 2 Adrenaline/Epinephrine (Noradrenaline/Norepinephrine)
- Chapter 3 Ammonium Nitrate
- Chapter 4 Artemisinin
- Chapter 5 Aspirin
- Chapter 6 Caffeine
- Chapter 7 Capsaicin
- Chapter 8 Carbon Dioxide
- Chapter 9 β-Carotene
- Chapter 10 Chlorophyll
- Chapter 11 Cholesterol
- Chapter 12 Cisplatin
- Chapter 13 Cocaine
- Chapter 14 DEET
- Chapter 15 Difluorodichloroethane, CF2Cl2: (Freon-12, CFC-12 or R-12) and Related Compounds
- Chapter 16 DDT
- Chapter 17 Digitalis
- Chapter 18 Dimethylmercury
- Chapter 19 Dimethylsulfide
- Chapter 20 Dopamine
- Chapter 21 Epibatidine
- Chapter 22 Estradiol
- Chapter 23 Glucose
- Chapter 24 Glycerol
- Chapter 25 Heavy Water: Deuterium Oxide, D2O
- Chapter 26 Heme
- Chapter 27 Hexenal
- Chapter 28 Hydrogen Peroxide
- Chapter 29 Insulin
- Chapter 30 Kisspeptin
- Chapter 31 Lauric Acid
- Chapter 32 Limonene
- Chapter 33 Linoleic Acid
- Chapter 34 Lysergic Acid Diethylamide (LSD)
- Chapter 35 Medroxyprogesterone Acetate
- Chapter 36 Methamphetamine
- Chapter 37 Methane
- Chapter 38 2-Methylundecanal
- Chapter 39 Monosodium Glutamate
- Chapter 40 Morphine, Codeine and Heroin
- Chapter 41 Nandrolone
- Chapter 42 Nicotine
- Chapter 43 Nitrous Oxide, N2O
- Chapter 44 1-Octen-3-ol
- Chapter 45 Oxygen (and Ozone)
- Chapter 46 Oxytocin
- Chapter 47 Paracetamol/Acetaminophen
- Chapter 48 Penicillins
- Chapter 49 Prostanoic Acid and Prostaglandins
- Chapter 50 Psilocybin and Mescaline
- Chapter 51 Quinine
- Chapter 52 Sodium Hypochlorite
- Chapter 53 Serotonin
- Chapter 54 Skatole
- Chapter 55 Sucrose
- Chapter 56 ‘Sweaty’ Acid, (E)-3-Methyl-2-Hexenoic Acid
- Chapter 57 Taxol (Paclitaxel)
- Chapter 58 Testosterone
- Chapter 59 Tetrahydrocannabinol (THC)
- Chapter 60 Tetrahydrogestrinone (THG)
- Chapter 61 Tetrodotoxin
- Chapter 62 Thujone
- Chapter 63 Trimethylamine
- Chapter 64 TNT
- Chapter 65 Vancomycin
- Chapter 66 VX Gas
- Chapter 67 Water
- Bibliography
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Molecules That Amaze Us by Paul May,Simon Cotton in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.