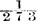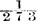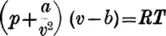![]()
ATOMIC THEORIES
INTRODUCTION: ATOMIC THEORIES: EARLIER VIEWS
DEMOCRITUS, at a time dating back to 400 B.C., gave expression to atomic ideas in connection with matter, etc., which in a general sense are true to-day. So striking are the ideas attributed to this philosopher that Tyndall mentioned them, and Millikan * quotes from Tyndall, as follows:—
“1. From nothing comes nothing. Nothing that exists can be destroyed. All changes are due to the combination and separation of molecules.
“2. Nothing happens by chance. Every occurrence has its cause from which it follows by necessity.
“3. The only existing things are the atoms and empty space; all else is mere opinion.
“4 The atoms are infinite in number and infinitely various in form; they strike together and the lateral motions and whirlings, which thus arise, are the beginnings of worlds.
“5. The varieties of all things depend upon the varieties of their atoms, in number, size and aggregation.
“6. The soul consists of fine, smooth, round atoms like those of fire. These are the most mobile of all. They interpenetrate the whole body and in their motions the phenomena of life arise.”
Professor Millikan remarks: “These principles with a few modifications and omissions might almost pass muster to-day.” Of course, the reader will appreciate that it is a far cry from such dreams to the present-day knowledge of matter and electricity.
The first definite step in the development of the atomic theory was made independently by William Higgins and John Dalton—the former in 1789 advanced the idea that each chemical compound was definite in the molecular sense. For instance, the oxides of nitrogen according to Higgins would be: NO, NO2, NO3, NO4 and NO5. Dalton in 1803 arrived at a similar conclusion whereby the nitrogen oxides would be N2O, NO and NO2.*
These results involved the conception of atoms combining in definite proportions, but Higgins regarded the 1 : 1 proportion as the more stable. It would appear that Dalton had no knowledge of the earlier views of Higgins, which were published in a book,† the title of which would not have invited Dalton’s attention. It is to Dalton that the credit of developing the theory properly belongs; hence his name has been rightly associated with it.
The atomic theory involves by analysis three principles, viz. (1) Definite proportions; (2) Multiple proportions; and (3) Equivalent proportions. In the first, the constituents of every given chemical compound are fixed and immutable; i.e. however formed, and wherever found, a particular compound is the same. It seems safe to remark here that no comprehensive statement can be made which is not liable to slight modification as more knowledge is gained. For example, the different species of lead (isotopes) differ slightly in atomic weight and, therefore, compounds involving these leads would not contain exactly equal proportionate amounts of lead, though all the compounds would bear the same name and they would be chemically identical. In the second, when one element combines with another, the proportions by weight are in multiples of the smallest proportion corresponding with the atom: in the sense that A+B=P; or 2A+B=Q; or 2A+3B=R, etc., P, Q, R, etc., representing compounds. In the third, each element in combining with or displacing another element follows a rule of fixed proportions by weight represented by a particular number, or a simple multiple or submultiple of that number, for each element involved, which represents the relative combining weight. The atomic weight then becomes a whole multiple or submultiple of its combining weight.
These regularities led to the establishment of the atomic theory involving definite weights and the conception of valence numbers. In this case the atomic weight divided by the combining weight represents the valence; or, knowing the valence integer and the combining or equivalent weight, these two quantities multiplied together give the atomic weight. These matters become clearer if approached with later knowledge, as will be seen from the following chapters.
Dalton regarded the atoms as indivisible particles which for a given element were similar to one another in respect of atomic weight. While this idea is true in the main, the presence of isotopes introduces a modification in the theory, as will be seen later.
The atom, moreover, cannot be defined as an unalterable entity, even in the chemical sense, for if a hydrogen atom can part with an electron and become thereby a positive ion, according to current theory, its character or composition is not, strictly speaking, preserved, as H plus an electron does not equal H minus an electron. Furthermore, recent experiments, as fully detailed in Chapter VIII, show that the atoms of some of the lighter elements can be partly disintegrated by means of a process of bombardment, the dismembered parts having masses of the order of 1, 2 and 3, hydrogen being taken as unity.
The atoms of Dalton have been regarded as the smallest particles of matter which take part in chemical combination, but there are other considerations which make this definition too narrow. The inert elements, such as helium, neon, argon, etc., do not enter into chemical combinations, yet they are made up of atoms. The electron, which is an atom of negative electricity, plays a profound part in the process of chemical combination, and it is also the active agent in electrical phenomena; and this entity has to be considered in chemical and in physical processes, as will be seen from what follows. The electron in various numbers appears to be an essential constituent of all atoms.
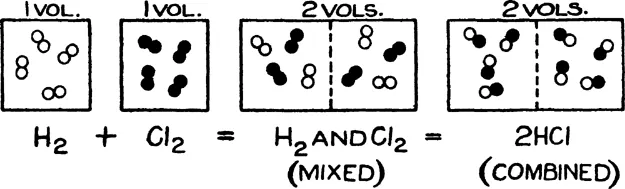
There are other phenomena besides the above which give expression to the principle of discontinuity of matter, and some of these lead to the reality of molecules. The kinetic theory of gases affords strong evidence that the molecular state of matter is one which is in harmony with the atomic basis of all compounds, for, in breaking up and manipulating gaseous bodies known to consist of molecules in rapid movement, the gas laws apply and in particular the law of Avogadro stating that the number of molecules in a given volume of any gas is the same when the pressure of the gas on the walls of the vessel is constant and its temperature is also constant. The law of Avogadro can be deduced from the kinetic theory of gases. In applying Avogadro’s law when decomposition of gaseous molecules takes place accompanied by recombination to form a different gaseous substance, the atomic theory becomes evident, as will be seen from the well-known example of Fig. 1.
There are five laws, not all of which are exact, that afford an interpretation of gas properties which is in harmony with the atomic theory. These may be briefly stated as follows:—
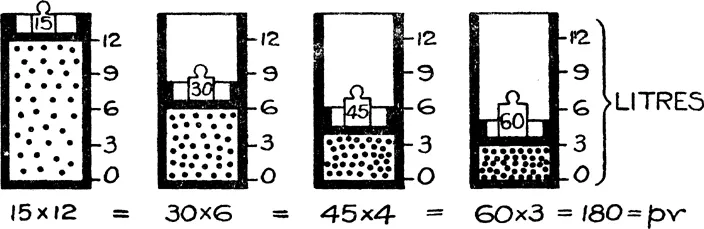
1. Boyle’s law states that the pressure (p) multiplied by the volume (v) of a given weight of gas is constant when the temperature is maintained constant: that is, pv=constant. The following diagram, Fig. 2, after one adopted by Mellor,* should make this law clearer.
As stated above, the gas laws are not exact, and to meet this difficulty van der Waals proposed an equation which gives the behaviour of the gas over a wide range of temperature and pressure. Before coming to van der Waals’ equation it will be desirable to proceed a little further.
2. The law of Charles or Gay-Lussac connecting the volume of a gas with its temperature states that under conditions of constant pressure the volume increases by
rd of its value at 0° C. for every 1° C. increase of temperature. This law can be stated so as to connect temperature and pressure, for, by keeping the volume constant, for each 1° C. rise of temperature the pressure increases
rd of that at 0° C.
This is not the place to discuss gas laws, since it belongs more properly to another branch of chemical physics, but van der Waals’ reasoning which was the basis of his famous equation is of particular interest in connection with the fundamental behaviour of atoms of which this book treats.
Starting with the general characteristics of all gases, viz. that the molecules are in themselves perfectly elastic and all the energy of a striking blow is returned to the particle as kinetic energy, i.e. energy of motion, the following factors have been taken into account by van der Waals:—
(i) The molecules have definite sizes, and larger molecules will make more to-and-fro excursions between opposite walls of the containing vessel than smaller ones, assuming in both cases their velocities to be the same. This will be quite obvious if the molecules are imagined to be very large on the one hand and very small on the other, the walls being the same distance apart in both cases. There is in consequence of the increase in molecular size an increase of pressure for, as just shown, more impacts per second take place. This may be expressed in terms of a contracted volume v — b, where b is a constant dependent upon the space occupied by the molecules.
(ii) A second correcting factor is based upon the supposition that the molecules attract one another appreciably at high pressures when they are closer together, and van der Waals assumed that the attraction was proportional to the product of the masses of the gas particles, or to the square of the density of the gas; this factor appears in the form a/v2, a being a constant which varies in passing from one kind of gas to another.
Combining these two factors the final equation becomes
in which
p=observed pressure of the gas.
v=measured volume of the gas.
a=constant as above ...