
Catalytic Asymmetric Reactions of Conjugated Nitroalkenes
- 302 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Catalytic Asymmetric Reactions of Conjugated Nitroalkenes
About This Book
Nitroalkenes have often been referred to as "synthetic chameleons" owing to their reactivity, synthetic utility and biological significance. In the last two decades, the reactivity of nitroalkenes as substrates in diverse catalytic asymmetric transformations has been of tremendous interest on account of the powerful abilities of the nitro group to coordinate and withdraw electrons, as well as its amenability to undergo a wide variety of synthetic transformations. Although numerous original articles and reviews have appeared in the literature, a monograph providing a comprehensive coverage of this topic was conspicuous by its absence.
This book features:
-
- A systematic, up-to-date, in-depth and well-organized compilation, spread over 12 chapters, of various catalytic asymmetric reactions of nitroalkenes with diverse substrates reported to date
-
- A wide coverage of reactions such as Michael additions, Friedel–Crafts reactions, cycloadditions, asymmetric reductions, multicomponent and cascade reactions, as well as other miscellaneous reactions
-
- Various chiral organo, metal and even biocatalysts involved in the stereoselective synthesis of multifunctional adducts via catalytic asymmetric reactions of nitroalkenes
-
- Schemes and figures detailing all the reagents, reaction conditions and product profiles
-
- Mechanistic details, including transition state models, which will be useful for effective catalytic design
This book will be an invaluable resource for those who are working in the area of asymmetric catalysis and synthetic methodologies.
Frequently asked questions
1 Catalytic Asymmetric Michael Addition of 1,3-Dicarbonyls to Nitroalkenes
1.1 Introduction
1.2 Addition of 1,3-Diketones
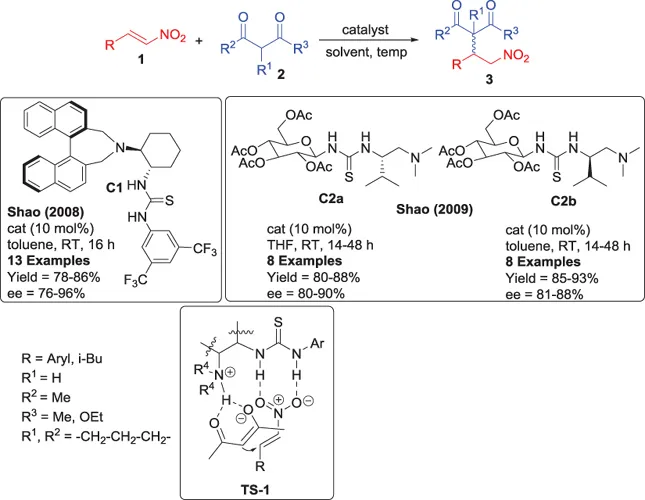
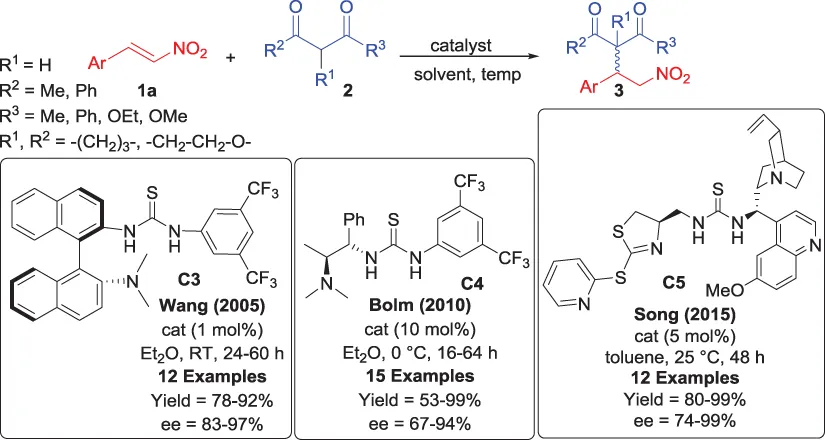
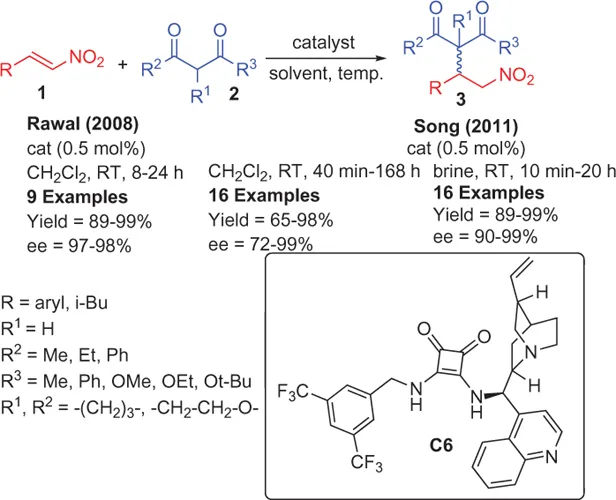
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- List of Abbreviations
- Foreword
- Preface
- Authors
- Introduction
- 1 Catalytic Asymmetric Michael Addition of 1,3-Dicarbonyls to Nitroalkenes
- 2 Catalytic Asymmetric Michael Addition of Aldehydes to Nitroalkenes
- 3 Catalytic Asymmetric Michael Addition of Ketones to Nitroalkenes
- 4 Catalytic Asymmetric Michael Addition of Miscellaneous Carbonyl Compounds to Nitroalkenes
- 5 Catalytic Asymmetric Friedel–Crafts Reactions of Nitroalkenes
- 6 Catalytic Asymmetric Michael Addition of Miscellaneous Carbon-centered Nucleophiles to Nitroalkenes
- 7 Catalytic Asymmetric Michael Addition of Heteroatom-centered Nucleophiles to Nitroalkenes
- 8 Catalytic Asymmetric Cycloadditions of Nitroalkenes
- 9 Catalytic Asymmetric Reduction of Nitroalkenes
- 10 Catalytic Asymmetric Synthesis of Cycloalkanes via Cascade Reactions of Nitroalkenes
- 11 Catalytic Asymmetric Synthesis of Aryl-Fused Heterocycles via Cascade Reactions of Nitroalkenes
- 12 Catalytic Asymmetric Synthesis of Five- and Six-Membered Heterocycles via Cascade Reactions of Nitroalkenes
- Index